Fig. 4.1
Illustration of epigenetic structure in the mature sperm and the dramatic organization that occurs in the early embryo immediately following fertilization. The top panel shows the protamine-bound mature sperm, undergoing chromatin decondensation marked by the removal of protamine proteins. The bottom panel shows the active demethylation that occurs in the paternal pronucleus, as well as the passive, replication-dependent demethylation that occurs in the maternal pronucleus
These observations were the first to suggest that the epigenetic status of sperm might be important for early development. While the mature sperm nucleus is comprised primarily of protamine-bound DNA, about 5 % of the DNA remains bound to histones (Hammoud et al. 2009). Until recently, it was unclear whether the persistent histones were the result of incomplete histone replacement or whether they served a functional purpose. Several years ago, our lab demonstrated that histones are consistently retained at specific loci including developmental gene promoters, genes encoding microRNAs, and imprinted loci. In addition, it was found that the retained histones often display bivalency, the presence of both activating and silencing modifications within the same region, which is reminiscent of stem cell signatures (Hammoud et al. 2009). These findings suggest that the epigenetic status of sperm is tightly regulated and likely mechanistically important for embryogenesis and early development. Following fertilization, the sperm nucleus undergoes decondensation and pronuclear development, and the protamines are replaced by oocyte-derived histones. During this process, the majority of DNA methylation marks are removed to restore totipotency to the sperm and oocyte genomes (Fig. 4.1), which clearly raises questions regarding the importance of pre-fertilization epigenetic marks; however, two important considerations are warranted. First, the identity of unmodified loci remains uncertain, raising the possibility that key sperm loci remain unchanged and functionally important during embryogenesis. Second, data suggest that epigenetic abnormalities in male gametes may affect embryo development, and there is evidence to suggest that these abnormalities can affect offspring phenotype. Even less data are available on the impacts of age-associated sperm epigenetic alterations and their impacts on the embryo. Despite this, there are many indications that age-associated epigenetic alterations may play a role in both embryogenesis and offspring health.
4.3 Delayed Parenthood
Advanced paternal age has recently become a heavily investigated topic as a result of multiple studies demonstrating ties between advanced paternal age and various offspring abnormalities. Additional trends contributing to the increasing interest in the role of advanced paternal age in reproduction is the trend in delayed parentage (Mills et al. 2011). Though this trend is justified by increasing life expectancies in both sexes, advanced paternal age may affect general semen parameters and sperm quality ultimately altering fecundity and offspring health. While many couples consider the risks associated with advanced maternal age in family planning decisions, very little thought has been given to the age of male partners. In recent history, paternal age has steadily increased, particularly in developed countries. This trend is believed to be associated with increased life expectancy, socioeconomic pressures, and divorce rates with subsequent remarriage at older ages (Kuhnert and Nieschlag 2004). During a 10-year span (1993–2003) in Great Britain, the percent of fathers within the age range of 35–54 increased from 25 % of total births to 40 %. Associated with this trend was a decrease in the number of births to fathers less than 35 years of age from 74 % of total births to only 60 % (Bray et al. 2006). Over two decades in Australia (1988–2008), the average age of fathers has increased by approximately 3 years (Australian Bureau of Statistics 2009). Similarly, the average age of fathers in Germany increased by 2 years over a 10-year period (Kuhnert and Nieschlag 2004). Congruent trends can be found in the United States and many other developed countries. As average paternal age continues to increase, it is becoming increasingly important to characterize the potential consequences of advanced paternal age on embryonic development and offspring health.
4.4 Heritability of Epigenetic Alteration Through the Paternal Germline
Though poorly understood, there is clear evidence that demonstrates a unique mechanism of heritability through the paternal germline. This idea initially became of great interest to many different scientific fields as a result of findings from growing catalogs of epidemiologic data coupled with landmark studies in mouse models. Specifically, data collected during and following massive crop failures in Sweden in the late 1800s and early 1900s was used to perform large retrospective studies in human populations. From these studies, it was found that paternal diet, independent of other factors, contributes to offspring disease susceptibility and general health in ways never before identified (Kaati et al. 2007; Pembrey et al. 2006). Though the nature of the data set made it impossible to understand any biological mechanisms that underlie these alterations, many believe it plausible that alterations of heritable epigenetic marks in gametes play some role in the process. In support of this idea is the work on the agouti viable yellow gene in mouse models, which demonstrated that nutrition can affect offspring phenotype through heritable altered epigenetic marks (Waterland and Jirtle 2003). This and other work have stimulated the study of transgenerational inheritance as we see it today.
Many intriguing studies have come as a result of the increased emphasis on transgenerational inheritance in the literature. One recent study found that male mice fed a low-protein diet, when mated with a normal female, sire offspring with altered expression of many genes important in metabolism and cholesterol synthesis (Carone et al. 2010). Similarly, metabolic alterations, specifically changes in insulin sensitivity, were also seen in the female offspring of male rats fed high-fat diets (Ng et al. 2010).
Although no concrete mechanism for inheritance of altered metabolic activity has been identified or any other nongenetic inheritance from the male germline, there are intriguing candidates including epigenetic inheritance through altered sperm DNA methylation. A recent study strongly supports the idea of transgenerational inheritance. Govorko et al. demonstrated that the offspring of male mice exposed in utero to alcohol had altered hypothalamic proopiomelanocortin (POMC) gene activity as a result of hypermethylation at the POMC promoter and that these deficits were passed down through the F3 generation (Govorko et al. 2012). Interestingly, although the methylation marks at the POMC promoter were similar in the F1 female and male (both exposed to prenatal alcohol), the alterations were not inherited via the maternal germline, suggesting a unique mechanism of epigenetic inheritance through the paternal germline (Govorko et al. 2012). Taken together, these data demonstrate the likelihood that the sperm epigenome plays an essential role in embryogenesis and is capable of contributing to offspring health.
4.5 Age-Associated Sperm Epigenetic Alterations
An important consideration in the role of paternal aging on embryo quality and offspring health is the degree to which the sperm is susceptible to genomic or epigenomic perturbation that could lead to embryonic or offspring dysfunction and disease. Because of the plastic nature of epigenetic marks in the sperm and the potential heritability of any perturbations, sperm epigenetics, in particular DNA methylation, has become one of the main candidates on which studies have focused. Only recently has data become available to describe the epigenetic landscape of the aged sperm, and these have focused primarily on DNA methylation in both human and mouse models. It is informative to describe this in context of somatic cell alteration associated with age where it is known that DNA methylation is altered in many somatic cell types with age in relatively consistent patterns (Wilson and Jones 1983; Oakes et al. 2003). From the few studies that have been performed, it appears that sperm methylation patterns resultant from aging are far different and of greater magnitude than what is seen in somatic cells (Jenkins et al. 2013, 2014). In fact, these cells display a virtually opposite profile of epigenetic change with age (Fig. 4.2). Although this may appear counterintuitive, it is important to note that other genomic alterations, such as telomere length, follow similar trends between these two tissue types (Eisenberg 2011). Furthermore, the idea that the magnitude of methylation alteration is greater in sperm as compared to somatic cells with aging is not without precedence. Work in support of this idea demonstrates that frequently dividing cells have more striking methylation changes associated with age than do cells that divide less frequently. As sperm undergo large amounts of division over the lifespan of an individual, it is not surprising that the magnitude of epigenetic change is greater in sperm over time than in other human tissues.
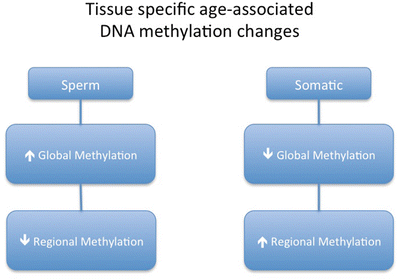

Fig. 4.2
General tissue-specific age-associated alterations that occur in sperm and other somatic tissues. Sperm tend to have slight increases in global methylation with age, while regionally there is a bias toward methylation loss. In somatic cells the opposite is true, as global methylation decreases and regional methylation increases with age
Two recent studies on human sperm from anonymous donors have revealed distinct patterns of methylation alteration with age (Jenkins et al. 2013, 2014). These studies utilized sperm donors who collected two samples many years apart (between 10 and 20 years approximately). This allowed the authors to analyze paired data to determine the intraindividual impact of aging on the sperm methylome. It was discovered that there is an increase in the global level of methylation in human sperm, a surprising finding based on the baseline hypermethylation in the mature sperm and the contrasting global hypomethylation that occurs with age in somatic cells (Jenkins et al. 2013, 2014). A number of regional alterations (approximately 1,000 bps in length) were also significantly altered with age and displayed a strong bias toward demethylation. This finding is, again, in opposition to what has been described in somatic cells where there is a bias toward regional hypermethylation (Jenkins et al. 2014). Alterations at these sites were confirmed with the use of targeted bisulfite sequencing in an independent cohort of unpaired general population sperm samples. These findings were remarkably consistent at the identified regions of alteration (Fig. 4.3). Intriguingly, it appeared that the age-associated regional alterations identified were enriched at genes known to be associated with neuropsychiatric disease. Similar results were identified in mice where regional hypomethylation was common in the sperm of aged mice though no global changes were identified (Milekic et al. 2014). Interestingly, all offspring of older males had similar alterations to methylation patterns in brain tissue coupled with alterations in social behaviors. Taken together, age-associated methylation perturbations represent a plausible mechanism by which the increased incidence of disease in the offspring of older fathers may be transmitted.
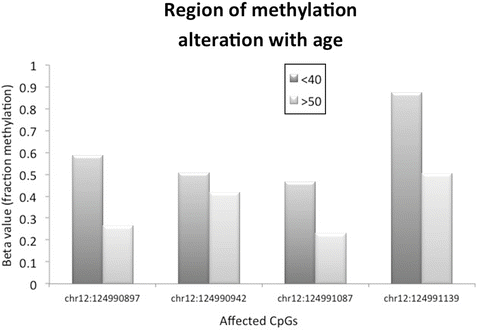

Fig. 4.3
An example of sperm-specific regional methylation. At this relatively small genomic window (approximate 250 bps), there is a significant decrease in fraction methylation (y axis) at each CpG (x axis) that occurs within men over 50 (n = 9) when compared to men under 40 (n = 12). These data represent an example of one of many loci significantly affected by age in (Jenkins et al. 2014)
4.6 Embryo Quality, Pregnancy Outcomes, and Offspring Health
The effects of paternal age on pregnancy outcome and embryo quality are controversial. This controversy is mainly a result of the scant data available on the subject. Some reports suggest that there is a significant decline in fertility (as measured by time to pregnancy) with age, while others report no such associations (Hassan and Killick 2003; Begueria et al. 2014). Additional data does suggest that paternal age is a significant factor when compounded with maternal age (de la Rochebrochard and Thonneau 2002). Other studies support these data by suggesting an increased frequency of fetal loss, increased time to pregnancy, and decreased probability of conception in older men (Selvin and Garfinkel 1976; Ford et al. 2000; Dunson et al. 2002). However, there are conflicting data which suggest little to no effect of paternal age on fertility in natural conception or with the use of assisted reproductive technologies (ART) (Begueria et al. 2014; Olsen 1990; Bellver et al. 2008). Similar controversy exists in the effect of paternal age on embryo quality with the use of ART with some studies showing no effect (Bellver et al. 2008; Ferreira et al. 2010) and some suggesting decreased quality of embryos sired by older fathers on day 3, 4, and 5 (Luna et al. 2009; Frattarelli et al. 2008). The most compelling indication that paternal age may affect embryo quality is data on miscarriage. In general, the consensus from the available data is that advanced paternal age is a risk factor for miscarriage though no real mechanisms for this finding have been elucidated (Kleinhaus et al. 2006; Slama et al. 2005). Other studies evaluating ART with donor eggs (to completely remove the influence of maternal factors) found no associations between paternal age and risk of miscarriage (Begueria et al. 2014). Taken together, much work remains to determine what, if any, effect advanced paternal age has on male fertility and embryo quality.
The subtlety of the effect of age on male fertility, and particularly pregnancy outcomes, is in striking contrast to the dramatic decline seen in female fertility. In fact, even men of advanced age are able to sire offspring with little difficulty, though possibly with slightly reduced efficiency which is why paternal age has largely been ignored and has received far less attention in the clinical setting than the age of the female partner. The fact that males are still fertile at advanced ages may present, and potentially complicate, another issue that, while subtle, is far more consistent, namely, the effect of paternal age on offspring health and disease susceptibility. It has been shown that the offspring of older fathers have increased incidence of various forms of cancer, including hematological and central nervous system tumors (Hemminki et al. 1999; Oksuzyan et al. 2012; Murray et al. 2002; Yip et al. 2006), though the data remains somewhat controversial. Furthermore, it has long been suggested that advanced paternal age is a risk factor for schizophrenia (Hare and Moran 1979; Miller et al. 2011; Matheson et al. 2011; Wohl and Gorwood 2007). More recently, it has been suggested that advanced paternal age is significantly associated with many forms of neuropsychiatric or neurocognitive diseases including autism spectrum disorders (ASD) (Gardener et al. 2009; Hultman et al. 2011), bipolar disorder (Frans et al. 2008; Menezes et al. 2010), and general increases in behavioral issues (Kuja-Halkola et al. 2012; Saha et al. 2009a) in children of older fathers though some controversy exists. In addition, some studies indicate that children of older fathers display slightly reduced IQ compared with children of younger fathers (Malaspina et al. 2005; Saha et al. 2009b), although the differences are small, and conflicting reports exist (Svensson et al. 2011).
Taken together, it is clear that advanced paternal age does not have a dramatic affect on pregnancy outcomes, embryo quality, or fertility in general, but it may impact offspring health and disease susceptibility. While the lack of striking age-associated fertility declines in males has garnered it little attention in the study of fertility, it is this same maintenance of fertility that might require more study in the field of transgenerational inheritance. Age-associated alterations to sperm, which appear to affect offspring health, do not seem to be catastrophic to spermatogenesis or cause declines in fertility. This, in turn, means that aged sperm are entirely competent to fertilize an oocyte and produce viable offspring, while harboring alterations that may potentially affect offspring health.
4.7 Future Directions
To gain a more complete understanding of the epigenetic alterations in sperm that are capable of embryonic or offspring phenotype alterations, much work is still needed. A number of genomic regions have been identified that have both methylation alterations with age and are important in various cell processes and diseases known to have increased occurrence in the offspring of older males. To determine if these marks can contribute to disease susceptibility in the offspring or affect events in the embryo, a number of unanswered questions must be addressed.
What is the impact of altered methylation profiles at our regions of interest? To completely understand the alterations which have been identified and their impact on offspring phenotype or embryo development, it must be determined if these alterations are associated with transcriptional changes. Future work can target genomic sites that are known to be altered with age in mouse models to determine if (1) there are changes to transcription in the embryo and (2) determine if there are altered transcript levels in various tissues in the offspring (should the sperm be competent to generate viable offspring).
Do the altered methylation marks seen in sperm escape, or impact in any way, embryonic epigenetic reprogramming? This is an essential question to fully understand the impact of an altered epigenetic profile. It is feasible that an alteration could affect embryogenesis in one of two ways. First, it could directly affect transcription of an important developmental factor. Second, the epigenetic abnormality may result in not a targeted perturbation but in the global alteration of epigenetic reprogramming, effectively altering an important aspect of embryogenesis, likely to the point of embryonic arrest.
Do methylation perturbations contribute to neuropsychiatric disorders in the offspring or perturbations to embryogenesis? To date, there are many intriguing studies that have provided some small degree of insight into the effect of aging on the sperm epigenome. However, much of the potential impacts are simply extrapolation of the available data without any real targeted studies to prove causative relationships. While the data is exciting, future targeted work is still required to enable us to reach these further conclusions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


