The Prostate and the Testis
THE PROSTATE
The purpose of cytologic or histologic sampling of the prostate is the diagnosis of prostatic carcinoma. In the past, the presumptive clinical diagnosis of prostatic cancer was based on finding palpable nodules on rectal examination. In 1930, Ferguson, working at the Memorial Hospital for Cancer in New York where the aspiration of various organs was extensively used, reported that cytologic examination of such nodules was diagnostically helpful and accurate.
Within the recent years, rectal examination is supplemented by testing for serum level of prostate specific antigen (PSA) which has become the principal means of detection of occult, localized carcinoma of the prostate (Catalona et al, 1991; Stenman et al, 1999; Brawer, 2000). Elevation of PSA, beyond the levels established for various age groups (4 to 6 μg/liter), is an indication for a transrectal ultrasound examination of the prostate that may reveal foci of abnormalities not recognizable by palpation (Cooner et al, 1990). The finding of such abnormal areas in the prostate is usually followed by either core or fine (thin) needle aspiration (FNA) biopsies to confirm the presence of prostatic carcinoma prior to treatment.
It should be stressed that elevation of the levels of PSA also depends on the volume of the prostate: it may be increased in benign prostatic hypertrophy and prostatitis, and may be transient (Lieber et al, 2001). For this reason, refinement of the PSA testing has been proposed within recent years. Thus, the ratio of free to protein bound PSA (specifically to α1-antichymotrypsin and sometimes other enzymes) is lower in prostatic carcinoma than in benign disease (Stenman et al, 1999). Prostatic carcinoma may also be associated with increased PSA density and velocity measured on multiple tests over a 3 year period (Polascik et al, 1999; Kamoi and Babaian, 1999; Nash and Melezinek, 2000). On the other hand, a small percentage of patients with prostatic carcinoma have normal PSA levels (Stamey and Kabalin, 1989).
The current aggressive approach to the treatment of
prostatic carcinoma by radical surgery or radiotherapy, often combined with chemotherapy, affecting ever younger men, disregards the fact that prostatic carcinoma is one of the most common forms of occult cancer found at autopsy of elderly men, reaching 80% in men aged 80 or above (Peterson, 1992). Thus, it is likely that at least some of the treated carcinomas would not be lethal to the patients within their natural life span. In fact, several long-term follow-up studies of patients with untreated, low-grade prostatic carcinoma have shown that they have a minimal risk of dying of the disease (Johansson et al, 1992, 1997; Albertsen, 1998).
prostatic carcinoma by radical surgery or radiotherapy, often combined with chemotherapy, affecting ever younger men, disregards the fact that prostatic carcinoma is one of the most common forms of occult cancer found at autopsy of elderly men, reaching 80% in men aged 80 or above (Peterson, 1992). Thus, it is likely that at least some of the treated carcinomas would not be lethal to the patients within their natural life span. In fact, several long-term follow-up studies of patients with untreated, low-grade prostatic carcinoma have shown that they have a minimal risk of dying of the disease (Johansson et al, 1992, 1997; Albertsen, 1998).
Although there are some fairly reliable prognostic factors, discussed below, to assess the behavior of prostatic carcinomas, they are rarely applied to treatment decisions in individual patients, many of whom believe that all prostate cancers are a rapidly progressing deadly disease. Equally disturbing is the absence of randomized trials, documenting that PSA testing does reduce mortality from prostatic carcinoma (Barry, 2001).
Be it as it may, extensive testing for PSA has raised the status of prostatic carcinoma from a relatively unimportant disease several years ago to the forefront of health care of men with 198,000 new cancers and 31,000 deaths from this disease projected by the American Cancer Society for the year 2001 (Greenlee et al, 2001). Men of African origin appear to be at a high risk for this disease which is relatively uncommon among the Japanese. Prostatic carcinoma can occur in families, but its causes and risk factors are generally unknown.
SAMPLING METHODS
Voided Urine
Cells of prostatic cancer may occasionally be observed in voided urine, as described in Chapter 23. The finding is usually incidental and reflects advanced disease.
Prostatic Massage
Prostatic massage is commonly used by urologists to obtain samples of prostatic secretions for rapid microscopic analysis, mainly for identification of leukocytes in cases of prostatitis. This method had been used to secure samples for diagnosis of prostatic carcinoma in patients with palpable prostatic abnormalities with a measure of success (Frank et al, 1954; Frank, 1955; Bamforth, 1958; Clarke and Bamford, 1960). However, the method is not suitable for detection of occult prostatic carcinoma, as was shown in a large study of over 2,000 asymptomatic patients aged 50 or older (Riaboff, 1954; Richardson et al, 1954).
Transrectal Aspiration Biopsy
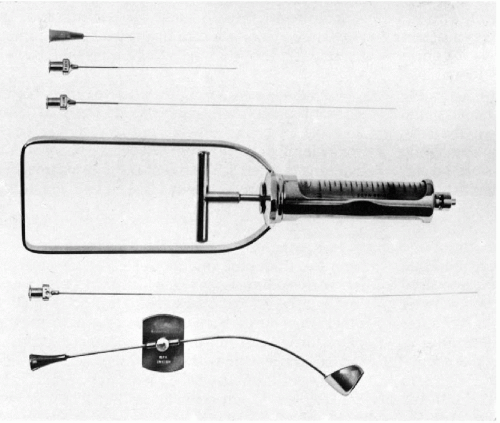
The development of a special instrument for prostatic aspiration (Franzén et al, 1960) led to an effective method of cytologic sampling of the prostate by transrectal fine (thin) needle aspiration biopsy (FNA) (Fig. 33-1). The scope, significance, and clinical value of the aspiration biopsy of the prostate has undergone significant changes since the late Dr. Josef Zajicek eloquently and enthusiastically described it in the previous editions of this book. The changes were caused by new developments in ultrasonographic techniques that are capable of identifying small space-occupying lesions of the prostate that can be sampled by core biopsy instruments that many clinicians find easier to use than the cumbersome aspiration apparatus. Further, pathologists with limited experience in diagnostic cytology find the core biopsies easier to interpret than aspiration cytology. Core biopsies offer the additional advantage of a more precise localization of the lesions within the target organ. Still, the FNA of the prostate offers several advantages:
It is an office procedure.
It is well tolerated by the patients because the discomfort and trauma from the 22-gauge needle are minimal.
Sampling area is larger than that of core biopsies.
Smears can be processed and interpreted rapidly.
It is accurate in experienced hands.
It is cost effective.
Complications are very rare and easily preventable.
Disadvantages
The main disadvantage of the aspiration is that it is limited to palpable lesions. Another disadvantage is the complexity of the instrument requiring considerable training, experience and clinical skills to perform the
aspiration quickly and effectively (Ljung et al, 2001). In the Montefiore Hospital experience, with 1,683 patients sampled by a group of practicing urologists or urology residents without specific training in the technique of aspiration, 37% of the samples were inadequate (Koss et al, 1984; Suhrland et al, 1988). Most American urologists have now abandoned the aspiration procedure in favor of the core tissue biopsy (see below). Still, the aspiration biopsy of the prostate is a useful and accurate diagnostic procedure, if the expensive ultrasound or core biopsy instruments are not available.
aspiration quickly and effectively (Ljung et al, 2001). In the Montefiore Hospital experience, with 1,683 patients sampled by a group of practicing urologists or urology residents without specific training in the technique of aspiration, 37% of the samples were inadequate (Koss et al, 1984; Suhrland et al, 1988). Most American urologists have now abandoned the aspiration procedure in favor of the core tissue biopsy (see below). Still, the aspiration biopsy of the prostate is a useful and accurate diagnostic procedure, if the expensive ultrasound or core biopsy instruments are not available.
An additional application of cytologic aspiration techniques to prostatic carcinoma was described by Van Poppel et al (1994). These investigators used computerized tomography-guided transcutaneous aspirations of pelvic lymph nodes to identify metastatic carcinoma with high sensitivity and specificity.
Aspiration Procedure using Franzén’s Instrument
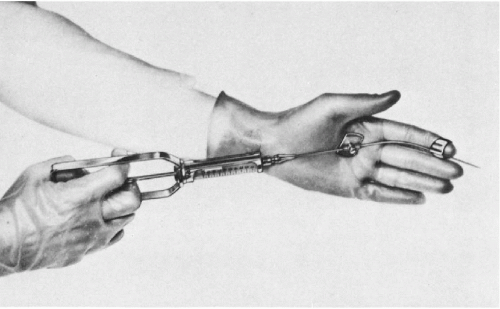
As shown in Figure 33-1, the aspiration of the prostate calls for a specially constructed 22-gauge, 20-cm in length flexible needle, which is slightly thicker and rigid in the proximal 5 cm. The transition from the thicker to the thinner part of the needle serves as a marker (see below). A needle guide, consisting of a fine metal tube curved to fit the palpating finger, is required (see Fig. 33-1, bottom). The length of the guide equals that of the thinner part of the needle. To facilitate introduction of the needle into the guide, the latter is enlarged at its proximal end like a funnel. At the distal end of the guide, there is a steering ring for the palpating finger. This may be adjusted to fit the operator’s finger. An adjustable plate, midway along the guide, serves to support the instrument by resting on the palm of the gloved nondominant (usually left) hand of the operator. The index finger of the gloved hand is passed through the steering ring, leaving the fingertip free. To fix the guide more firmly on the palpating finger and to avoid contamination, a finger-cot is thereafter pulled over the index finger. The support plate is adjusted to rest on the palm of the hand and kept in place by pressure of the remaining fingers flexed against the palm. The needle is introduced through the funnel into the guide until the thicker part of the needle approaches the funnel. At that moment, the needle point is nearing the end of the guide immediately below the finger-cot (Fig. 33-2).
The aspiration is performed with the patient in lithotomy position. No anesthesia is required, except in patients with inflamed hemorrhoids or anal fistula who receive an anesthetic jelly. Careful digital examination of the lesion to assess its size and consistency is first made. The left index finger, with the instrument arranged as shown in Figure 33-3, is then inserted into the rectum. The finger must be well lubricated and must be inserted slowly and carefully, as this may be the most uncomfortable moment for the patient, particularly if there is an anal spasm.
The suspected area of the prostate is now palpated with the index finger of the nondominant hand, after which the needle is advanced into the lesion with the plunger of the syringe down. When the needle has entered the lesion, several small amplitude to-and-fro movements of the needle are performed to loosen the target tissue. Negative pressure is obtained by pulling on the syringe plunger in order to aspirate the material onto the needle. Before withdrawing the needle from the prostate, the negative pressure must be released, a most important step that will ensure that the aspirated material remains in the needle and does not enter the barrel of the syringe, where it is irretrievably lost. If several areas of the prostate require aspiration, the needle has to be withdrawn and replaced with another needle. It is not advisable to attempt to change the direction of the needle, while lodged in the target tissue, because of risk of a hemorrhage and injury to the prostate. The smears are prepared from needle contents, as described in Chapter 28, and processed as either air-dried or alcohol-fixed smears. Most patients tolerate the procedure well. Significant discomfort was reported by only a few patients with acute prostatitis and prostatic carcinoma. Repeat aspirations were readily accepted by the patients.
Although the procedure described above was initially devised by Franzén et al for prostatic aspiration, it is also applicable to the sampling of the ovary and the parametria (see Chap. 15).
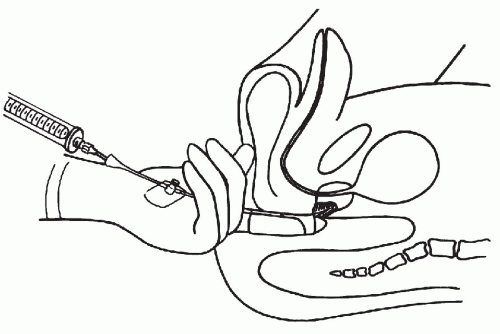 Figure 33-3 Position of the left hand during transrectal aspiration biopsy. Prostate in sagittal section showing the needle inserted into the suspected, indurated area. (Franzén S, et al. Cytologic diagnosis of prostatic tumors by transrectal aspiration biopsy: A preliminary report. Br J Urol 32:193-196, 1960.) |
Complications
In the approximately 17,000 transrectal prostatic aspiration biopsies initially performed at the Karolinska Hospital in Stockholm, Sweden between 1956 and 1966, no major complications were observed. Minor complications, such as transient hematuria, acute epididymitis, hematospermia or transient pyrexia, occurred in 0.4% of patients.
In the subsequent years, however, when the number of needle biopsies increased sharply, there were four cases of severe, gram-negative septicemia, one with a fatal outcome (Esposti et al, 1975). It was noted that the febrile reactions were more common in patients with prostatitis, reaching 6.3% of patients in some groups. The obvious conclusion was that transrectal aspiration biopsy should not be undertaken in patients with current prostatitis. It is still a matter of debate whether prophylactic treatment with antibiotics is effective in preventing septicemia. Because of the remote possibility of septicemia, every patient undergoing a transrectal aspiration biopsy of the prostate should be advised to contact his own physician or the nearest hospital should a febrile reaction occur. However, a past history of prostatitis does not, in itself, contraindicate an aspiration biopsy because 10% in this group of patients were found in the Swedish study to have carcinoma.
Core Biopsies
Today, most urologists prefer multiple core biopsies performed with a semi-automated instrument under ultrasound guidance for diagnosis of prostatic carcinoma. The method requires no anesthesia and is usually performed transrectally as an outpatient procedure. Tissue biopsies, obtained from six to twelve areas of the prostate are, not only diagnostic, but are also predictive of stage and grade of carcinoma (Wills et al, 1998). The accuracy of this procedure in the diagnosis of prostatic carcinoma is similar to that of expertly performed aspiration biopsies (see below for comparison of results of the two procedures). The complications of core biopsies are similar to those observed with the FNA procedure and include gram-negative septicemia that may be fatal.
ANATOMY AND HISTOLOGY OF THE PROSTATE AND SEMINAL VESICLES
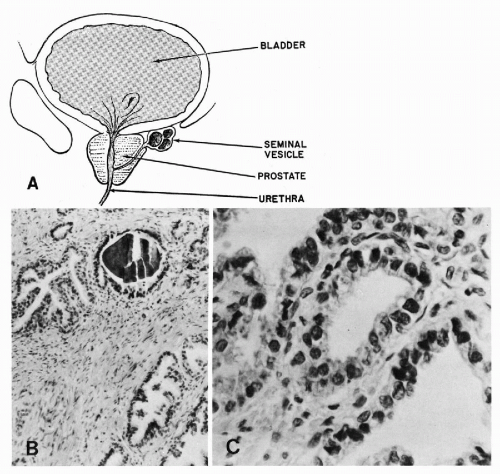
The prostate is a complex gland encased in a pseudocapsule of fibromuscular tissue (Fig. 33-4A). The prostatic glands, or acini, and ducts are lined by cuboidal to columnar epithelium with small, spherical nuclei. Nucleoli are absent, or very small, and barely visible under the high power of the microscope (Fig. 33-4B). The normal prostatic glands are provided with layers of basal epithelial cells that can be demonstrated by immunostaining with antibodies to high molecular weight keratins (keratin 903). This layer is usually absent in prostatic carcinoma, a feature that is sometimes helpful in differentiating atypical benign
prostatic epithelium from well differentiated adenocarcinoma (Hedrick and Epstein, 1989; Shah et al, 1991).
prostatic epithelium from well differentiated adenocarcinoma (Hedrick and Epstein, 1989; Shah et al, 1991).
It is a matter for some debate whether the prostatic glands are surrounded by myoepithelial cells which are extremely difficult to visualize in histologic sections. However, there is no doubt that small, spindly, dark cells, recognized in other organs as myoepithelial cells, can be seen in prostatic aspiration smears.
Within the lumina of the glands, one may frequently observe concretions of prostatic secretions (corpora amylacea) that may undergo calcification and thereby form “prostatic calculi” (Fig. 33-4B).
The sexual apparatus of men also includes two symmetric glandular structures—the seminal vesicles, attached to the posterior aspect of the prostate (see Fig. 33-4C). The seminal vesicles are in close relationship with the spermatic ducts, with which they share a common opening in the prostatic urethra. The seminal vesicles are lined by columnar cells, characterized by accumulation of a yellow lipochrome pigment in the cytoplasm. These cells are also notable for marked variability in nuclear sizes; in some cells, the nuclei are markedly enlarged and hyperchromatic (Arias-Stella and Takano-Moron, 1958). Spermatozoa and their precursors are stored within the lumens of the seminal vesicles.
CYTOLOGY OF NORMAL PROSTATE
Aspiration Biopsy
The microscopy of prostatic smears should begin at low magnification. In benign conditions, there is usually a thin film of blood and fluid with a small number of sheets of epithelial cells. If the aspirate has been properly spread on the slide, most of the prostatic cell clusters will be found at the end of the slide. An adequate smear will contain at least 10 to 12 clusters of epithelial cells.
Prostatic Epithelial Cells
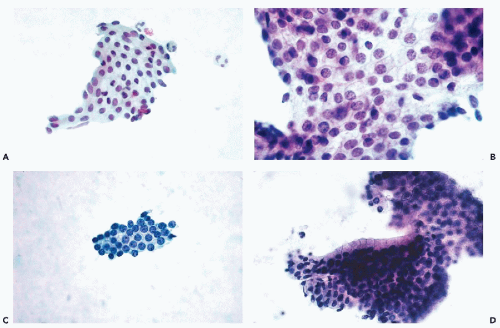
Normal prostatic epithelial acinar cells form flat sheets of uniform small cells with clear cytoplasm and small, spherical nuclei of even sizes. The cytoplasmic borders are readily seen as thin lines separating the cells from each other (honeycomb effect)




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree