Chapter Twelve. The placenta, membranes and amniotic fluid
Introduction
To survive and grow during intrauterine life the embryo is essentially in a parasitic relationship with the mother (Carlson 2004). It obtains nutrients and oxygen and disposes of waste products through the complex organ called the placenta. This organ is the defining characteristic of all mammals and is a key feature to the transfer of information from mother to fetus and vice versa. It has developed to ensure that the fetus is well protected during its development but problems and malfunctions can arise. By understanding the development and function of the placenta treatments to save babies lives may be possible (Gluckman & Hanson 2005).
Implantation
The embryo obtains nutrients and oxygen and disposes of waste products via its mother’s circulation through the placenta. The placenta is derived from embryonic trophoblast cells and a few inner cell mass mesodermal cells. The initial trophoblastic cells called collectively the cytotrophoblast give rise to the syncytiotrophoblast (trophoblast without cells) that has undergone nuclear division without forming daughter cells. This structure invades the uterine lining allowing the embryo to embed by the 10th day when a plug of blood clot and cellular debris closes over its point of entry. The syncytiotrophoblast at the embryonic pole of the zygote forms a thick multinucleated layer.
Maternal endometrial capillaries surrounding the embryo swell to form sinusoids which are eroded by the invasive syncytiotrophoblast. Small spaces called lacunae appear in the syncytiotrophoblast and become filled with a mixture of blood from the sinusoids and secretions from the eroded endometrial glands. This fluid is the embryotroph and passes to the embryonic disc by diffusion (Carlson 2004, Moore & Persaud 2008). The lacunae fuse to form the intervillous spaces of the placenta through which maternal blood begins to flow.
In normal pregnancy decidual and myometrial arteries undergo changes to convert them to uteroplacental arteries. Two types of migratory cytotrophoblast (MC) cause this:
1. Endovascular MC invades spiral arterioles on the decidua and myometrium and replaces arterial endothelium, destroying muscle and elastic tissues in the tunica media. The tissues are replaced by maternal fibrinoid. The migration takes place in two waves: at 6–10 weeks and at 14–16 weeks.
2. Interstitial (stromal) MC destroys the ends of decidual blood vessels, promoting blood flow into the lacunae. The maternal arteries are functionally denervated so that they are completely dilated and unresponsive to pressor substance or autonomic neural control. Local prostacyclin maintains vasodilation of the uterine radial arteries.
Soon mesodermal tissue from the developing embryo migrates through the primitive streak and joins trophoblast extensions to form the connecting stalk. This mesoderm gives rise to the umbilical blood vessels and the structure becomes the umbilical cord. By the end of the second week trophoblastic cells have formed finger-like projections called primary chorionic villi all round the embryo. The embryo, its umbilical vesicle and the early amniotic sac are suspended in the chorionic sac which consists of a layer of mesoderm nearest the embryo, the cytotrophoblast and the syncytiotrophoblast nearest the endometrium. The amniotic sac is nearest to the uterine wall and is divided from the chorion by a fluid-filled cavity, the extraembryonic coelom.
Development of the chorionic villi
Early in the 3rd week a core of loose connective tissue developed from embryonic mesenchyme invades each primary chorionic villus to form the secondary chorionic villi. Some of the mesenchymal cells in the core differentiate into fetal blood capillaries, forming mature tertiary chorionic villi. By 15–20 days there is a functioning arteriocapillary venous network connected to the embryonic heart vessels. By the end of the 3rd week fetal blood circulates through the chorionic villi and an exchange of substances between maternal and fetal circulations begins (Figure 12.1 and Figure 12.2).
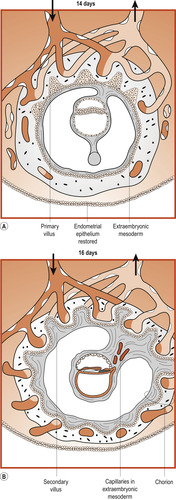 |
| Figure 12.1 Formation of chorionic vesicle. (From Fitzgerald M J T, Fitzgerald M 1994, with permission.) |
 |
| Figure 12.2 (A) Chorionic vesicle at 21 days. (B) Enlargement of the upper part of (A) showing the circulation of embryonic and maternal blood. (From Fitzgerald M J T, Fitzgerald M 1994, with permission.) |
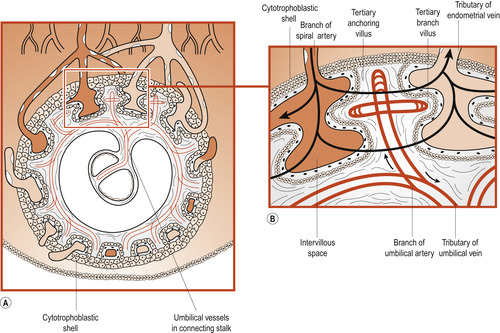
Formation of the cytotrophoblastic shell
At the same time the cytotrophoblastic cells proliferate and extend through the syncytiotrophoblast to form a cytotrophoblastic shell. This attaches the chorionic sac to the maternal endometrium by specialised chorionic villi called stem or anchoring villi. From the sides of the stem villi branch villi grow through and it is here that the main exchange of materials between maternal and fetal circulations occurs.
Until about 20 weeks the placental membrane (Fig. 12.3) consists of four layers separating the two circulations: syncytiotrophoblast, cytotrophoblast, connective tissue of the mesenchyme core and the endothelium of the fetal capillary (Moore & Persaud 2008). Maternal and fetal circulations do not mingle unless there is damage to the villi. As pregnancy advances, this placental membrane becomes thinner and many fetal capillaries lie very close to the syncytiotrophoblast.
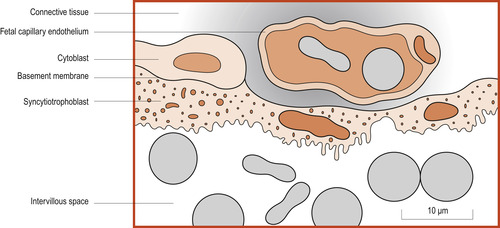 |
| Figure 12.3 Structure of the placental membrane. All erythrocytes are coloured grey. (From Fitzgerald M J T, Fitzgerald M 1994, with permission.) |
Later placental development
Once the conceptus has implanted, the decidual reaction spreads outwards from the embedding site and the endometrium becomes the decidua because it is shed at the end of pregnancy. Three regions of the decidua based on their relation to the implantation site are described:
1. The decidua basalis lies beneath the conceptus, forming the maternal component of the placenta.
2. The decidua capsularis overlies the conceptus.
3. The decidua vera or parietalis is the name of the remaining uterine lining.
As the conceptus grows the decidua capsularis bulges into the uterine cavity and eventually fuses with the decidua vera. By 22 weeks the decidua capsularis has degenerated and disappeared (Fig. 12.4).
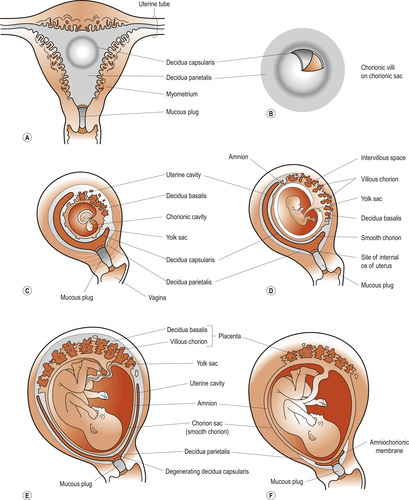 |
| Figure 12.4 (A) Drawing of a frontal section of the uterus showing the elevation of the decidua capsularis caused by the expanding chorionic sac of an implanted 4-week embryo. (B) Enlarged drawing of the implantation site shown in (A); the chorionic villi have been exposed by cutting an opening in the decidua capsularis. (C–F) Drawings of sagittal sections of the decidua. In (F) the amnion and chorion are fused with each other and the decidua parietalis, thus obliterating the uterine cavity. Note that the chorionic villi persist only where the chorion is associated with the decidua basalis; here they have formed the villous chorion. (From Moore K, Persaud T V N 2008, with permission.) |
The entire surface of the chorionic sac is covered by villi until the 8th week. As the sac grows the chorionic villi associated with the decidua capsularis become compressed, reducing their blood supply and degenerate to become the chorion laeve (smooth) which becomes the chorionic membrane. The chorionic villi of the decidua basalis branch to form the chorion frondosum or fetal part of the placenta. By 16 weeks the placenta reaches its full thickness and no new lobes or stem villi develop. Circumferential growth continues with branching of villi. The size and number of maternal capillaries increases, as does the surface area for gas exchange. Cellular proliferation stops at 35 weeks but cellular hypertrophy continues until term and there is scope for the fetus to signal to the placenta if its needs are not being met (Gluckman & Hanson 2005).
The mature placenta
Appearance
The placenta is a flattened discoid organ about 20 cm in diameter. It is 2.5 cm thick at the centre and thins out towards its circumference where it is continuous with the chorion. It is about one-sixth of the baby’s weight at term. There are two distinct surfaces (Fig. 12.5): the maternal surface attached to the decidua and the fetal surface covered with amnion into which the umbilical cord inserts.
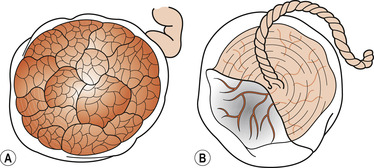 |
| Figure 12.5 The placenta. (A) The maternal surface, showing cotyledons, (B) the fetal surface. (From Henderson C, Macdonald S 2004, with kind permission of Elsevier.) |
At delivery the maternal surface is dark red because it contains maternal blood. Part of the decidua basalis will have been separated with it during delivery. The surface is formed of about 20 cotyledons (lobes) separated by sulci (grooves). The decidua dips down into these sulci to form septa. The lobes are made up of lobules, each containing a tertiary villus and its branches.
Because of the presence of the amnion the fetal surface is a shiny greyish white. From the insertion of the umbilical cord, branches of the single umbilical vein and two arteries spread out and dip down into the tissue. The amnion can be peeled off the surface, leaving the chorionic plate which is the portion continuous with the chorion.
The membranes
The amnion and chorion grow until about 28 weeks and then increase their size by stretching. They resist rupture as the fetus grows mainly due to the strength of the amnion. Rupture of the membranes during labour is probably brought about by increased intrauterine pressure as contractions reduce the intrauterine space and the amniotic fluid cannot be compressed. The amnion and chorion are not fused and contain up to 200 ml of amniotic fluid between them.
The outer chorion adheres closely to the decidua but the amnion moves over it aided by mucus. This may lead to rupture of the amnion with the formation of amniotic bands which may constrict or amputate fetal limbs (Blackburn 2007). The chorion is a thick, opaque, friable membrane which varies at term from 0.02 to 0.2 mm thick. It consists of four layers of tissue (Fig. 12.6) which atrophy as pregnancy advances. The cells of the chorion laeve are metabolically active, producing enzymes that can reduce the level of locally produced progesterone and a protein that can bind progesterone. The chorion also produces prostaglandins, oxytocin and platelet-activating factor, stimulators of myometrial activity.
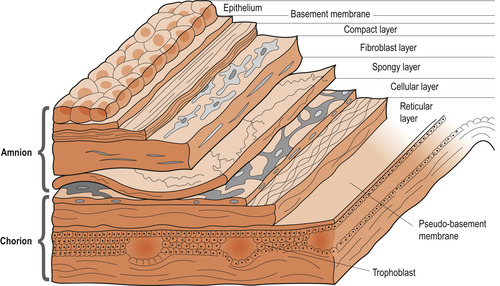 |
| Figure 12.6 Layers of the human amnion and chorion. (Reproduced with permission from Blackburn & Loper 1992.) |
The inner amnion is tough, smooth and translucent and lines the chorion and the surface of the placenta, continuing over the outer surface of the umbilical cord. At term it is about 0.02–0.5 mm thick and consists of five layers (Fig. 12.6). It is lined with non-ciliated epithelial cells which may help in the formation and regulation of amniotic fluid. The amnion also produces prostaglandins, in particular PGE 2, which may help to initiate the onset of labour. A rising ratio between estradiol and progesterone may regulate the activity of prostaglandin which may play an important part in the onset of labour by increasing the number of myometrial oxytocin receptors (Johnson 2007).
The umbilical cord
The umbilical cord or funis is usually attached to the centre of the placental fetal surface. It is 1–2 cm in diameter and varies in length from 30 cm to 90 cm with an average of 50 cm. There are normally two umbilical arteries and one umbilical vein surrounded by a mucoid connective tissue called Wharton’s jelly. The umbilical vein is longer than the arteries which spiral round it. The vessels are longer than the cord and non-significant loops of vessel called false knots may be seen. Rarely a true knot may be present and the blood vessels may become occluded, causing fetal distress, especially during labour.
The umbilical vesicle (yolk sac) and allantois
By 9 weeks the umbilical vesicle has shrunk to a pear-shaped remnant about 5 mm in diameter. Once its functions in producing blood cells during weeks 3–5 are completed, it becomes detached from the gut and remains present in the umbilical cord (Coad & Dunstall 2005). The allantois, which is an important structure for exchange of gases and removal of urinary waste but is never a prominent structure in the human embryo as in birds and some mammals (Carlson 2004), degenerates forming the urachus (median umbilical ligament) that connects the umbilicus to the urinary bladder. In 2% of adults the urachus persists as a Meckel’s diverticulum of the ileus.
The placental circulation
The placental villi form a huge surface for substance exchange between maternal and fetal circulations. Maternal blood enters the intervillous space in spurts via 80–100 endometrial spiral arteries. It flows slowly over the surface of the villi and substances are exchanged in both directions. The maternal blood reaches the floor of the intervillous space where it drains into the endometrial veins (Figure 12.7, Figure 12.8 and Figure 12.9). Anything interfering with the uteroplacental circulation will result in fetal hypoxia and may interfere with growth or cause death. An analogy for the placental circulation is taking a shower. If you stand under the shower, your body is the tertiary villus, the shower head is the spiral arteriole delivering blood and the shower drainage is the endometrial vein.
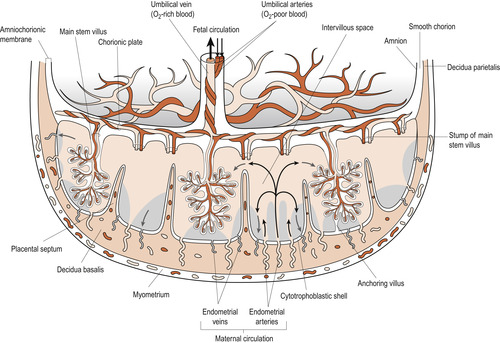 |
| Figure 12.7 Schematic drawing of a transverse section through a full-term placenta, showing: the relation of the villous chorion (fetal part of the placenta) to the decidua basalis (maternal part of the placenta; the fetal circulation; and the maternal placental circulation). Maternal blood flows into the intervillous spaces in funnel-shaped spurts from the spiral arteries, and exchanges of material between the mother and the embryo/fetus occur. The inflowing arterial blood pushes venous blood out of the intervillous space into the endometrial veins, which are scattered over the entire surface of the decidua basalis. Note that the umbilical arteries carry poorly oxygenated fetal blood (shown in dark grey) to the placenta and that the umbilical vein carries oxygenated blood (shown in light grey) to the fetus. Note also that the cotyledons are separated from each other by placental septa, projections of the decidua basalis. Each cotyledon consists of two or more main stem villi and their many branches. In this drawing only one stem villus is shown in each cotyledon but the stumps of those that have been removed are indicated. (Reproduced from Moore N 1989, with permission.) |
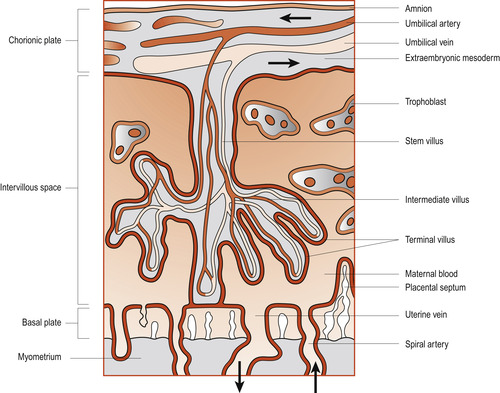 |
| Figure 12.8 Diagrammatic section through the placenta. The arteries carry deoxygenated blood; the veins carry oxygenated blood. Arrows indicate direction of blood flow. (From Fitzgerald M J T, Fitzgerald M 1994, with permission.) |
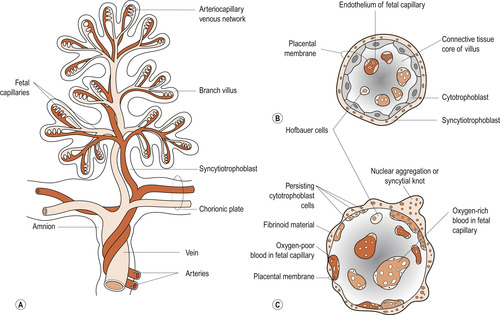 |
| Figure 12.9 (A) Drawing of a stem chorionic villus showing its arteriocapillary venous system. The arteries carry poorly oxygenated fetal blood and waste products from the fetus, whereas the vein carries oxygenated blood and nutrients to the fetus. (B, C) Drawings of sections through a branch villus at 10 weeks and full term, respectively. The placental membrane, composed of extrafetal tissues, separates the maternal blood in the intervillous space from the fetal blood in the capillaries in the villi. Note that the placental membrane becomes very thin at full term. Hofbauer cells are thought to be phagocytic. (Reproduced from Moore N 1989, with permission.) |
Deoxygenated blood leaves the fetus and passes into the two umbilical arteries which take it to the placenta. Here within the chorionic villi the fetal blood is brought very close to maternal blood from which it picks up oxygen. The oxygenated blood enters the umbilical vein which returns it to the fetus. The fetal circulation is described in detail in Chapter 48.
Anatomical variations of the placenta
Placentas may be abnormally shaped and it is important to examine the placenta and seek medical aid if it appears incomplete.
Succenturiate lobe
Sometimes a separate placental lobe is linked by blood vessels to the main placenta. Failure to deliver this succenturiate lobe may lead to infection and haemorrhage. Each placenta must be checked for a hole in the membranes with blood vessels leading away from it (Fig. 12.10).
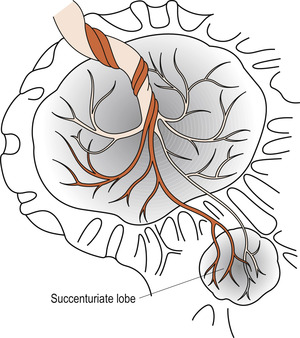 |
| Figure 12.10 Succenturiate placenta. (From Henderson C, Macdonald S 2004, with kind permission of Elsevier.) |
Battledore placenta
The umbilical cord is inserted into the edge of the placenta, giving it the appearance of a battledore (a bat used to play a medieval game similar to badminton).
Velamentous insertion of the cord
The umbilical cord insertion is into the membranes outside the placental boundary. Rarely the umbilical vessels cross the internal os, a condition known as vasa praevia. If these vessels rupture during labour, the fetus may have a massive haemorrhage.
Circumvallate placenta
An opaque thickened ridge is seen on the fetal surface of the placenta which forms because of doubling back of the membranes (Fig. 12.11). The membranes may leave the placenta nearer to the centre than normal. It is associated with an increased risk of growth retardation.
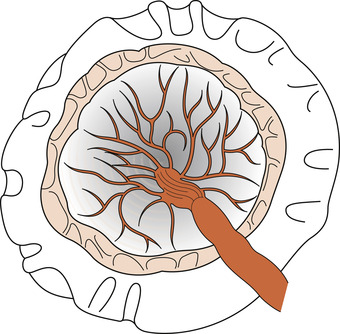 |
| Figure 12.11 Circumvallate placenta.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|