OBJECTIVES
After studying this chapter, you should be able to:
Describe the structure of the pituitary gland and how it relates to its function.
Define the cell types present in the anterior pituitary and understand how their numbers are controlled in response to physiologic demands.
Understand the function of hormones derived from proopiomelanocortin in humans, and how they are involved in regulating pigmentation in humans, other mammals, and lower vertebrates.
Define the effects of the growth hormone in growth and metabolic function, and how insulin-like growth factor I (IGF-I) may mediate some of its actions in the periphery.
List the stimuli that regulate growth hormone secretion and define their underlying mechanisms.
Understand the relevance of pituitary secretion of gonadotropins and prolactin, and how these are regulated.
Understand the basis of conditions where pituitary function and growth hormone secretion and function are abnormal, and how they can be treated.
INTRODUCTION
The pituitary gland, or hypophysis, lies in a pocket of the sphenoid bone at the base of the brain. It is a coordinating center for control of many downstream endocrine glands, some of which are discussed in subsequent chapters. In many ways, it can be considered to consist of at least two (and in some species, three) separate endocrine organs that contain a plethora of hormonally active substances. The anterior pituitary secretes thyroid-stimulating hormone (TSH, thyrotropin), adrenocorticotropic hormone (ACTH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin, and growth hormone (see Figure 17–9), and receives almost all of its blood supply from the portal hypophysial vessels that pass initially through the median eminence, a structure immediately below the hypothalamus. This vascular arrangement positions the cells of the anterior pituitary to respond efficiently to regulatory factors released from the hypothalamus. Of the listed hormones, prolactin acts on the breast. The remaining five are, at least in part, tropic hormones; that is, they stimulate secretion of hormonally active substances by other endocrine glands or, in the case of growth hormone, the liver and other tissues (see below). The tropic hormones for some endocrine glands are discussed in the chapter on that gland: TSH in Chapter 19; and ACTH in Chapter 20. However, the gonadotropins FSH and LH, along with prolactin, are covered here.
The posterior pituitary in mammals consists predominantly of nerves that have their cell bodies in the hypothalamus, and stores oxytocin and vasopressin in the termini of these neurons, to be released into the bloodstream. The secretion of these hormones, as well as a discussion of the overall role of the hypothalamus and median eminence in regulating both the anterior and posterior pituitary, was covered in Chapter 17. In some species, there is also a well-developed intermediate lobe of the pituitary, whereas in humans it is rudimentary. Nevertheless, the intermediate lobe, as well as the anterior pituitary, contains hormonally active derivatives of the proopiomelanocortin (POMC) molecule that regulate skin pigmentation, among other functions (see below). To avoid redundancy, this chapter will focus predominantly on growth hormone and its role in growth and facilitating the activity of other hormones, along with a number of general considerations about the pituitary. The melanocyte-stimulating hormones (MSHs) of the intermediate lobe of the pituitary, α-MSH and β-MSH, will also be touched upon.
MORPHOLOGY
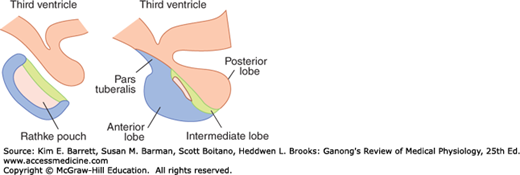
The anatomy of the pituitary gland is summarized in Figure 18–1 and discussed in detail in Chapter 17. The posterior pituitary is made up largely of the endings of axons from the supraoptic and paraventricular nuclei of the hypothalamus and arises initially as an extension of this structure. The anterior pituitary, on the other hand, contains endocrine cells that store its characteristic hormones and arises embryologically as an invagination of the pharynx (Rathke pouch). In species where it is well developed, the intermediate lobe is formed in the embryo from the dorsal half of Rathke pouch, but is closely adherent to the posterior lobe in the adult. It is separated from the anterior lobe by the remains of the cavity in Rathke pouch, the residual cleft.
In the posterior lobe, the endings of the supraoptic and paraventricular axons can be observed in close relation to blood vessels. Pituicytes, stellate cells that are modified astrocytes, are also present.
As noted above, the intermediate lobe is rudimentary in humans and a few other mammalian species. In these species, most of its cells are incorporated in the anterior lobe. Along the residual cleft are small thyroid-like follicles, some containing a little colloid (see Chapter 19). The function of the colloid, if any, is unknown.
The anterior pituitary is made up of interlacing cell cords and an extensive network of sinusoidal capillaries. The endothelium of the capillaries is fenestrated, like that in other endocrine organs. The cells contain granules of stored hormone that are extruded from the cells by exocytosis. Their constituents then enter the capillaries to be conveyed to target tissues.
Five types of secretory cells have been identified in the anterior pituitary by immunocytochemistry and electron microscopy. The cell types are the somatotropes, which secrete growth hormone; the lactotropes (also called mammotropes), which secrete prolactin; the corticotropes, which secrete ACTH; the thyrotropes, which secrete TSH; and the gonadotropes, which secrete FSH and LH. The characteristics of these cells are summarized in Table 18–1. Some cells may contain two or more hormones. It is also notable that the three pituitary glycoprotein hormones, FSH, LH, and TSH, while being made up of two subunits, all share a common α subunit that is the product of a single gene and has the same amino acid composition in each hormone, although their carbohydrate residues vary. The α subunit must be combined with a β subunit characteristic of each hormone for maximal physiologic activity. The β subunits, which are produced by separate genes and differ in structure, confer hormonal specificity (see Chapter 16). The α subunits are remarkably interchangeable and hybrid molecules can be created. In addition, the placental glycoprotein gonadotropin, human chorionic gonadotropin (hCG) has α and β subunits (see Chapter 22).
The anterior pituitary also contains folliculostellate cells that send processes between the granulated secretory cells. These cells produce paracrine factors that regulate the growth and function of the secretory cells discussed above. Indeed, the anterior pituitary can adjust the relative proportion of secretory cell types to meet varying requirements for different hormones at different life stages. This plasticity has recently been ascribed to the presence of a small number of pluripotent stem cells that persist in the adult gland.
PROOPIOMELANOCORTIN & DERIVATIVES
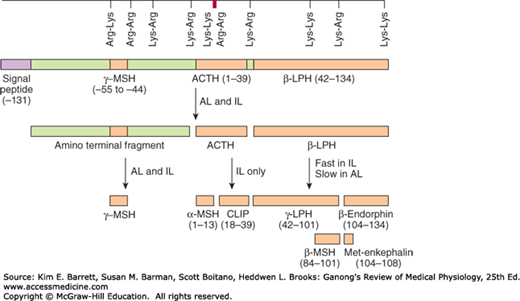
Intermediate-lobe cells, if present, and corticotropes of the anterior lobe both synthesize a large precursor protein that is cleaved to form a family of hormones. Removal of the signal peptide results in the formation of the prohormone POMC. This molecule is also synthesized in the hypothalamus, the lungs, the gastrointestinal tract, and the placenta. The structure of POMC, as well as its derivatives, is shown in Figure 18–2. In corticotropes, it is hydrolyzed to ACTH and β-lipotropin (β-LPH), plus a small amount of β-endorphin, and these substances are secreted. In the intermediate lobe cells, POMC is hydrolyzed to corticotropin-like intermediate-lobe peptide (CLIP), γ-LPH, and appreciable quantities of β-endorphin. The functions, if any, of CLIP and γ-LPH are unknown, whereas β-endorphin is an opioid peptide (see Chapter 7) that has the five amino acid residues of met-enkephalin at its amino terminal end. The melanotropins α- and β-MSH are also formed. However, the intermediate lobe in humans is rudimentary, and it appears that neither α-MSH nor β-MSH is secreted in adults. In some species, however, the melanotropins have important physiologic functions, as discussed below.
FIGURE 18–2
Schematic representation of the preproopiomelanocortin molecule formed in pituitary cells, neurons, and other tissues. The numbers in parentheses identify the amino acid sequences in each of the polypeptide fragments. For convenience, the amino acid sequences are numbered from the amino terminal of ACTH and read toward the carboxyl terminal portion of the parent molecule, whereas the amino acid sequences in the other portion of the molecule read to the left to –131, the amino terminal of the parent molecule. The locations of Lys–Arg and other pairs of basic amino acids residues are also indicated; these are the sites of proteolytic cleavage in the formation of the smaller fragments of the parent molecule. ACTH, adrenocorticotropic hormone; AL, anterior lobe; IL, intermediate lobe; LPH, lipotropin; MSH, melanocyte-stimulating hormone.
Fish, reptiles, and amphibia change the color of their skin for thermoregulation, camouflage, and behavioral displays. They do this in part by moving black or brown granules into or out of the periphery of pigment cells called melanophores. The granules are made up of melanins, which are synthesized from dopamine (see Chapter 7) and dopaquinone. The movement of these granules is controlled by a variety of hormones and neurotransmitters, including α- and β-MSH, melanin-concentrating hormone, melatonin, and catecholamines.
Mammals have no melanophores containing pigment granules that disperse and aggregate, but they do have melanocytes, which have multiple processes containing melanin granules. Melanocytes express melanotropin-1 receptors. Treatment with MSHs accelerates melanin synthesis, causing readily detectable darkening of the skin in humans in 24 h. As noted above, α- and β-MSH do not circulate in adult humans, and their function is unknown. However, ACTH binds to melanotropin-1 receptors. Indeed, the pigmentary changes in several human endocrine diseases are due to changes in circulating ACTH. For example, abnormal pallor is a hallmark of hypopituitarism. Hyperpigmentation occurs in patients with adrenal insufficiency due to primary adrenal disease. Indeed, the presence of hyperpigmentation in association with adrenal insufficiency rules out the possibility that the insufficiency is secondary to pituitary or hypothalamic disease because in these conditions, plasma ACTH is not increased (see Chapter 20). Other disorders of pigmentation result from peripheral mechanisms. Thus, albinos have a congenital inability to synthesize melanin. This can result from a variety of different genetic defects in the pathways for melanin synthesis. Piebaldism is characterized by patches of skin that lack melanin as a result of congenital defects in the migration of pigment cell precursors from the neural crest during embryonic development. Not only the condition but also the precise pattern of the loss is passed from one generation to the next. Vitiligo involves a similar patchy loss of melanin, but the loss develops progressively after birth secondary to an autoimmune process that targets melanocytes.
GROWTH HORMONE
The long arm of human chromosome 17 contains the growth hormone-hCS cluster that contains five genes: one, hGH-N, codes for the most abundant (“normal”) form of growth hormone; a second, hGH-V, codes for the variant form of growth hormone (see below); two code for human chorionic somatomammotropin (hCS) (see Chapter 22); and the fifth is probably an hCS pseudogene.
Growth hormone that is secreted into the circulation by the pituitary gland consists of a complex mixture of hGH-N, peptides derived from this molecule with varying degrees of posttranslational modifications, such as glycosylation, and a splice variant of hGH-N that lacks amino acids 32–46. The physiologic significance of this complex array of hormones has yet to be fully understood, particularly since their structural similarities make it difficult to assay for each species separately. Nevertheless, there is emerging evidence that, while the various peptides share a broad range of functions, they may occasionally exert actions in opposition to one another. hGH-V and hCS, on the other hand, are primarily products of the placenta, and as a consequence are only found in appreciable quantities in the circulation during pregnancy (see Chapter 22).
The structure of growth hormone varies considerably from one species to another. Porcine and simian growth hormones have only a transient effect in the guinea pig. In monkeys and humans, bovine and porcine growth hormones do not even have a transient effect on growth, although monkey and human growth hormones are fully active in both monkeys and humans. These facts are relevant to public health discussions surrounding the presence of bovine growth hormones (used to increase milk production) in dairy products, as well as the popularity of growth hormone supplements, marketed via the Internet, with body builders. Controversially, recombinant human growth hormone has also been given to children who are short in stature, but otherwise healthy (ie, without growth hormone deficiency), with apparently limited results.
A portion of circulating growth hormone is bound to a plasma protein that is a large fragment of the extracellular domain of the growth hormone receptor (see below). It appears to be produced by cleavage of receptors in humans, and its concentration is an index of the number of growth hormone receptors in the tissues. Approximately 50% of the circulating pool of growth hormone activity is in the bound form, providing a reservoir of the hormone to compensate for the wide fluctuations that occur in secretion (see below).
The basal plasma growth hormone level measured by radioimmunoassay in adult humans is normally less than 3 ng/mL. This represents both the protein-bound and free forms. Growth hormone is metabolized rapidly, at least in part in the liver. The half-life of circulating growth hormone in humans is 6–20 min, and the daily growth hormone output has been calculated to be 0.2–1.0 mg/d in adults.
The growth hormone receptor is a 620-amino-acid protein with a large extracellular portion, a transmembrane domain, and a large cytoplasmic portion. It is a member of the cytokine receptor superfamily, which is discussed in Chapter 3. Growth hormone has two domains that can bind to its receptor, and when it binds to one receptor, the second binding site attracts another, producing a homodimer (Figure 18–3). Dimerization is essential for receptor activation.
FIGURE 18–3
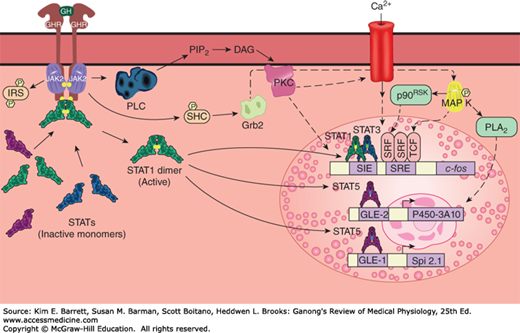
Some of the principal signaling pathways activated by the dimerized growth hormone receptor (GHR). Solid arrows indicate established pathways; dashed arrows indicate probable pathways. The details of the PLC pathway and the pathway from Grb2 to MAP K are discussed in Chapter 2. The small uppercase letter Ps in yellow hexagons represent phosphorylation of the factor indicated. GLE-1 and GLE-2, interferon γ-activated response elements; IRS, insulin receptor substrate; p90RSK, an S6 kinase; PLA2, phospholipase A2; SIE, Sis-induced element; SRE, serum response element; SRF, serum response factor; TCF, ternary complex factor.
Growth hormone has widespread effects in the body (see below), so even though it is not yet possible precisely to correlate intracellular and whole body effects, it is not surprising that, like insulin, growth hormone activates many different intracellular signaling cascades (Figure 18–3). Of particular note is its activation of the JAK2–STAT pathway. JAK2 is a member of the Janus family of cytoplasmic tyrosine kinases. STATs (for signal transducers and activators of transcription) are a family of cytoplasmic transcription factors that, upon phosphorylation by JAK kinases, migrate to the nucleus where they activate various genes. JAK–STAT pathways are known also to mediate the effects of prolactin and various other growth factors.
In young animals in which the epiphyses have not yet fused to the long bones (see Chapter 21), growth is inhibited by hypophysectomy and stimulated by growth hormone. Chondrogenesis is accelerated, and as the cartilaginous epiphysial plates widen, they lay down more bone matrix at the ends of long bones. In this way, stature is increased. Prolonged treatment of animals with growth hormone leads to gigantism.
When the epiphyses are closed, linear growth is no longer possible. In this case, an overabundance of growth hormone produces the pattern of bone and soft tissue deformities known in humans as acromegaly. The sizes of most of the viscera are increased. The protein content of the body is increased, and the fat content is decreased (Clinical Box 18–1).
Growth hormone is a protein anabolic hormone and produces a positive nitrogen and phosphorus balance, a rise in plasma phosphorus, and a fall in blood urea nitrogen and amino acid levels. In adults with growth hormone deficiency, recombinant human growth hormone produces an increase in lean body mass and a decrease in body fat, along with an increase in metabolic rate and a fall in plasma cholesterol. Gastrointestinal absorption of Ca2+ is increased. Na+ and K+
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree