The Normal Female Genital Tract
ANATOMY
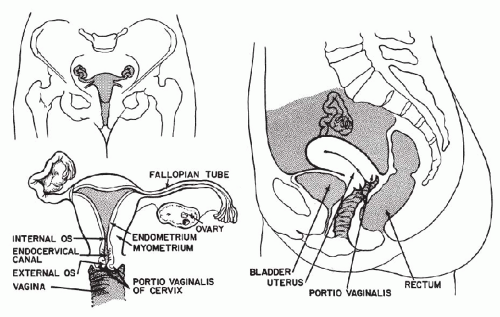
The female genital tract is composed of the vulva, the vagina, the uterus, the fallopian tubes, and the ovaries (Fig. 8-1).
Embryologic Note
The fallopian tubes, the uterus, and the adjacent part of the vagina are derived from two embryonal structures, the müllerian ducts, so named after Johannes Müller, a German anatomist of the early 19th century who first described them. The müllerian ducts fuse to become the uterus and the proximal vagina but remain separated to form the two oviducts (fallopian tubes). Imperfect fusion of the müllerian ducts results in formation of various degrees of duplication or subdivision of the uterus and the vagina, such as uterus septus and vagina septa. An excellent discussion of embryologic origin and congenital abnormalities of the female genital tract may be found in the book by Gray and Skandalakis (1972).
The Vulva
The vulva is the external portal of entry to the female genital tract. It is composed of two sets of folds or labia (from Latin, labium = lip; plural, labia), which frame both sides of the entrance to the vagina. The larger external folds, or labia majora (from Latin, majus = larger; plural, majora) are an extension
of the skin. The smaller inner folds, or labia minora (from Latin, minor = lesser; plural, minora), form a transition between the skin and the vagina. The outer surfaces of the labia minora retain some features of the skin, such as the presence of sebaceous glands, whereas the inner surfaces blend with the vagina. Located anteriorly between the labia minora is the female counterpart of the penis, the clitoris, provided with a retractile, prepuce-like structure. Located about 1 cm behind the clitoris is the opening of the urethra, the terminal portion of the urinary tract.
of the skin. The smaller inner folds, or labia minora (from Latin, minor = lesser; plural, minora), form a transition between the skin and the vagina. The outer surfaces of the labia minora retain some features of the skin, such as the presence of sebaceous glands, whereas the inner surfaces blend with the vagina. Located anteriorly between the labia minora is the female counterpart of the penis, the clitoris, provided with a retractile, prepuce-like structure. Located about 1 cm behind the clitoris is the opening of the urethra, the terminal portion of the urinary tract.
The lymphatic drainage of the vulva is to the inguinal lymph nodes, which are the primary site of metastases in malignant tumors of the vulva.
The Vagina
In virgins, the entrance to the vagina is protected by a thin, perforated membrane, the hymen. The torn hymen persists in the form of small vestigial elevations at the entrance to the vagina. Just behind the vestigial hymen, on both sides of the posterior and lateral aspect of the vagina, there are two mucus-secreting glands, the glands of Bartholin or Bartholin’s glands. During the childbearing age, the adult vagina is a canal, measuring approximately 10 cm in length, demarcated externally by the vulvar folds or labia, described above. The posterior end of the vagina is a blind pouch, the cul-de-sac. The anterior wall of the vagina, near the cul-de-sac, accommodates the uterine cervix. The area demarcated by the cervix and the blind end of the vaginal pouch is the posterior vaginal fornix. The fornix is quite deep and is the site wherein the secretions from the uterine glands, as well as exfoliated epithelial cells, accumulate. The wall of the vagina consists of three layers: the inner or mucosal layer of squamous epithelium, which shows transverse ridges or rugae. The mucosa is supported by a layer of smooth muscle. The thin outer serosal layer of the vagina is composed of connective tissue. The wall of the vagina is rich in lymphatic vessels. The lymphatic drainage of the anterior one-third of the vagina goes to the inguinal lymph nodes, whereas the posterior two-thirds drain into the pelvic lymph nodes.
Of importance are the anatomic relationships of the vagina, which are separated by thin connective tissue partitions or septa from the rectum posteriorly and the bladder anteriorly. Inflammatory processes and cancers of one of these organs may spread to the vagina and vice versa.
One of the rare but important congenital abnormalities of the vagina is vagina septa, in which the vagina, and possibly the uterus as well, is divided into two separate chambers. On occasion, this is of significance in tumor diagnosis, since cancer may be present in one part of the genital tract while the healthy part is being investigated with negative results.
The Uterus
The uterus is arbitrarily divided into two parts—the body, or corpus, and the neck, or cervix. The corpus and the cervix usually form an angle of 120°, with the corpus directed anteriorly. The body or corpus of the uterus is a roughly pyramidal organ, shaped like an inverted pear and flattened in the anteroposterior diameter. In the resting stage, it measures 4 to 7 cm in length and approximately the same at its widest point. The apex of the pyramid, which becomes the cervix, is directed downward, whereas the wide base, or fundus, is directed upward. The cervix is a tubular structure measuring approximately 4 cm in length and about 3 cm in diameter. Of its total length, about half is within the vagina and is called the portio vaginalis (also known as ecto- or exocervix); the rest is embedded within the vaginal wall and is continuous with the body of the uterus.
The bulk of the uterus is formed by smooth muscle, or the myometrium, which is capable of a manifold increase in size and weight during pregnancy. The muscle encloses the uterine cavity, described below, and is covered on its surface by a reflection of the peritoneum, known as the uterine serosa. The uterus is anchored in the pelvis by a series of bands of connective tissue, or ligaments, the most important being the
posterior round ligament, and by folds or reflections of the peritoneum. Lateral folds, extending along the sides of the uterus and filled with loose connective tissue rich in lymphatics, are known as the broad ligaments forming the left and the right parametrium (plural, parametria).
posterior round ligament, and by folds or reflections of the peritoneum. Lateral folds, extending along the sides of the uterus and filled with loose connective tissue rich in lymphatics, are known as the broad ligaments forming the left and the right parametrium (plural, parametria).
The cervix has a very close anatomic relationship to the urinary bladder, which is anterior, and to both ureters, which run along the lateral walls of the cervix to reach the bladder. This anatomic arrangement explains the frequent involvement of the lower urinary tract by cervical cancer.
The Uterine Cavity
The thick, muscular walls of the uterus contain a cavity that, within the cervix, is called the endocervical canal and is continuous with the endometrial cavity of the corpus. The opening of the cervical canal into the vagina is referred to as the external os (from Latin, os = mouth). The point of transition of the endocervical canal into the endometrial cavity is known as the internal os. The endocervical canal is normally very narrow, measuring at the most 2 or 3 mm in diameter. The endometrial cavity follows the outline of the body of the uterus and is roughly conical, with the apex of the cone corresponding to the internal os and the base directed upward to the upper part, or fundus, of the uterine body. On each side of the triangular endometrial cavity, the horns, or the cornua, of the fundus are in communication with the fallopian tubes, or the oviducts. The lumen of the endometrial cavity in the resting stage is quite small, measuring only a few millimeters in the anteroposterior diameter. The endometrial cavity during pregnancy enlarges to harbor the fetus.
The Fallopian Tubes
The fallopian tubes (so named after Gabriello Fallopius, an Italian anatomist of the 16th century, who first described them), or the oviducts, measure between 8 and 12 cm in length and 3 and 5 mm in diameter. Their proximal ends are in direct continuation with the endometrial cavity, whereas their distal ends, with fingerlike folds, or fimbriae, open freely into the abdominal cavity, embracing the ovaries. The ova, released by the ovaries, find their way into the fallopian tubes, where they are fertilized by spermatozoa. The tubes are composed of three layers—the inner mucosal layer, followed by a layer of smooth muscle, and a serosal layer on the surface. A narrow canal, lined by the mucosa, is present throughout the entire length of the tube, thereby ensuring direct communication between the vagina and the abdominal cavity—a fact of some importance in the spread of infections and malignant tumors. The histology of the fallopian tubes is discussed in Chapter 15.
Ovaries
The ovaries are approximately ovoid structures, each measuring on the average 4 by 2 by 2 cm, located anatomically in the immediate vicinity of the abdominal or fimbriated end of the tubes, but not directly contiguous with the tubal lumens. In spite of this, the ova, formed in the ovary, find their way into the tubes and from there into the uterine cavity. The ovaries are loosely suspended, as are the tubes, by peritoneal folds. The histology of the ovaries is discussed in Chapter 15.
Adnexa and Lymphatic Drainage
The term adnexa or uterine adnexa is used to describe, as a single entity, the structures peripheral to the uterus, which consist of the fallopian tubes, ovaries, parametria, and regional lymph nodes. The lymphatics of the uterus, the tubes, and the ovaries are the tributaries of the pelvic and the aortic lymph nodes.
HISTOLOGY OF THE UTERUS
Cytologic examination of the female genital tract is based mainly on the study of epithelial cells, with cells of other derivation playing only a minor role. Three types of epithelia are present within the uterus and the vagina: (1) the nonkeratinizing squamous epithelium that lines the inner aspect of the labia minora of the vulva, the vagina, and the portio vaginalis of the cervix; (2) the endocervical mucosa; and (3) the endometrium. All these epithelia, but especially the endometrium and the squamous epithelium, are under hormonal influence. The fullest development of these epithelia occurs during the childbearing age, and our description will be based on their appearance at this time. Subsequently, the changes observed in prepubertal and postmenopausal women will be described. Further details on the histology of the vulva and vagina are provided in Chapter 15.
Nonkeratinizing Squamous Epithelium
Squamous epithelium of the female genital tract is of two different embryologic origins. The epithelium lining the inner aspect of the labia minora and contiguous with the adjacent vagina, presumably to the level of the cervix, originates from the urogenital sinus. The remainder of the vaginal epithelium and the squamous epithelium of the vaginal portio of the cervix are derived from the müllerian ducts by transformation (metaplasia) of the original cuboidal epithelium into squamous epithelium. This fact has considerable bearing on certain congenital, neoplastic, and drug-induced abnormalities in the vagina and the cervix. The original squamous epithelium, not derived from metaplasia, is sometimes referred to as native squamous epithelium.
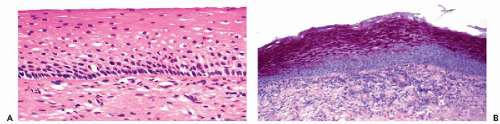
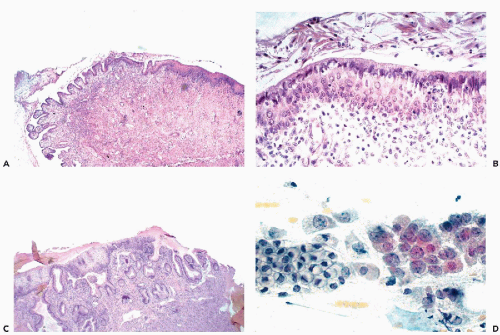
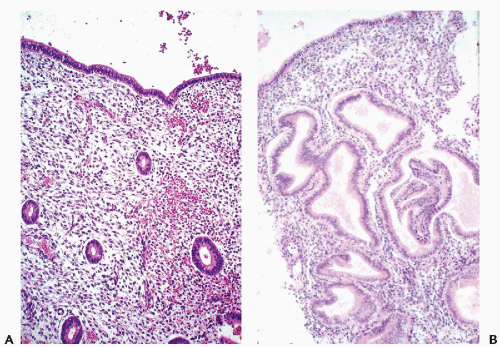
The fundamental structure of the squamous epithelium is described in Chapter 5 (see Fig. 5-4). In the female genital tract, during sexual maturity, four layers or zones may be arbitrarily discerned and include the bottom, or basal, layer, which is the source of epithelial regeneration; the adjacent parabasal zone, imperceptibly blending with the intermediate zone, forming the bulk of the epithelial thickness; and the thin superficial zone (Fig. 8-2A). It is estimated that the process of squamous epithelial maturation
takes approximately 4 days. The process may be accelerated to 30 to 45 hours by the administration of estrogens. The mature squamous epithelium of the cervix and vagina is rich in glycogen, as documented by periodic acid-Schiff stain (Fig. 8-2B). Clinically, the presence of glycogen may be revealed by staining the squamous epithelium with Lugol’s iodine solution, which, by binding with glycogen, stains the epithelium mahogany brown. This is the basis of Schiller’s test, which serves to visualize nonstaining, pale areas of the epithelium suggestive of an abnormality that can be either benign or malignant.
takes approximately 4 days. The process may be accelerated to 30 to 45 hours by the administration of estrogens. The mature squamous epithelium of the cervix and vagina is rich in glycogen, as documented by periodic acid-Schiff stain (Fig. 8-2B). Clinically, the presence of glycogen may be revealed by staining the squamous epithelium with Lugol’s iodine solution, which, by binding with glycogen, stains the epithelium mahogany brown. This is the basis of Schiller’s test, which serves to visualize nonstaining, pale areas of the epithelium suggestive of an abnormality that can be either benign or malignant.
The Epithelial Layers
The basal, or germinative, layer is composed of one row of small, elliptical cells, measuring approximately 10 μm in diameter. The vesicular nuclei, about 8 μm in diameter, commonly display evidence of active cellular growth, such as nucleoli or numerous chromocenters, and occasional mitoses. Under normal circumstances, the entire process of epithelial regeneration is confined to the basal layers; the remaining zones merely serving as stages of cell maturation.
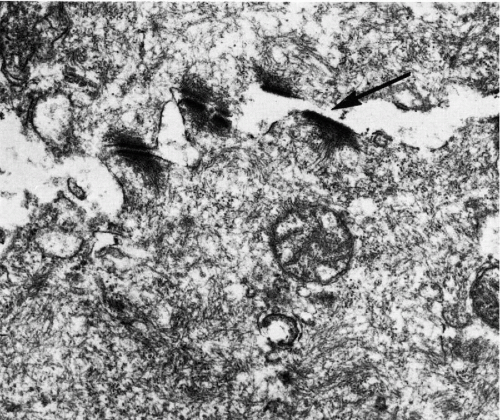
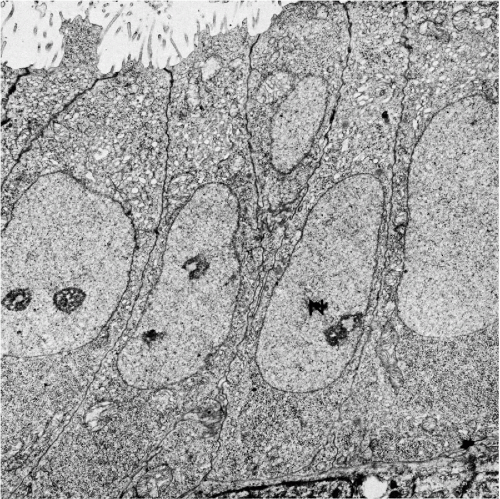
The wide midzone of the epithelium, comprising the parabasal and intermediate layers, is composed of maturing squamous cells. As the maturation of the epithelium progresses toward the surface, the amount of cytoplasm per cell increases, whereas the sizes of the vesicular nuclei remain fairly constant, measuring about 8 μm in diameter. Arbitrarily, the two or three layers of smaller cells of the deeper portion of the midzone are designated as parabasal layers. The larger cells, adjacent to the superficial zone, form the intermediate cell layers. If further maturation is arrested under various circumstances, the midzone may form the surface of the squamous epithelium. The cells forming the bulk of the epithelium are bound to each other by welldeveloped desmosomal attachments or intercellular bridges (Fig. 8-3).
The superficial zone is composed of three or four layers of loosely attached cells that are still larger than intermediate cells. The nuclei of the cells forming the surface of the epithelium are considerably smaller and pyknotic, measuring about 4 μm in diameter. These cells are not capable of further growth. The most superficial cells of the squamous epithelium are cast off the epithelial surface by a mechanism known as desquamation or exfoliation. The exfoliation either pertains to single squamous cells or to cell clusters. Within the clusters, the cells are still bound by desmosomes, as shown by electron microscopy (Dembitzer et al, 1976). The desquamation (exfoliation) of the squamous cells is related to splitting of the desmosomal bonds and, presumably, other cell attachments by an unknown mechanism (Fig. 8-4). It must be noted that, in vitro, the disruption of desmosomes among exfoliated squamous cells by either proteolytic enzymes or mechanical means, without destruction of the cells, is exceedingly difficult. Hence, one can only speculate either that specific enzyme systems become activated in the superficial layers of the epithelium or that intracytoplasmic changes occur that weaken the desmosomes and thereby allow the superficial cells to be dislodged, presumably by the pressure exercised by the growing epithelium.
The squamous epithelium is provided with an immune apparatus, represented by bone marrow-derived modified macrophages or dendritic cells, which are dispersed in the basal and central layers. Among the dendritic cells are the Langerhans’ cells, characterized by clear cytoplasm and vesicular nucleus. With special staining procedures, the branching cytoplasm of these cells can be identified (Figueroa and Caorsi, 1980; Roncalli et al, 1988). In electron microscopy, the cells are characterized by the presence of typical cytoplasmic tennis racquet-shaped granules, known as Birbeck’s granules (Younes et al, 1968). Edwards and Morris (1985) studied the distribution of the Langerhans’ cells in the squamous epithelium of the various parts of the female genital tract and found the highest concentration in the vulva and the lowest in the vagina. The Langerhans’ cells play an important role in the immune functions of the squamous epithelium.
The development of a superficial horny keratin layer composed of anucleated, fully keratinized cells, as observed in the epidermis of the skin (see Chap. 5), does not normally take place in the female genital tract but may occur under abnormal circumstances (see Chap. 10). On the other hand, in a variety of conditions (e.g., pregnancy, menopause, hormonal deficiency, inflammation), the squamous
epithelium may fail to reach its full maturity. In such cases, the surface of the squamous epithelium may be formed by intermediate or, sometimes, parabasal layers.
epithelium may fail to reach its full maturity. In such cases, the surface of the squamous epithelium may be formed by intermediate or, sometimes, parabasal layers.
Basement Membrane and the Supporting Apparatus
Immediately underneath the basal layer of the epithelium, there is a thin band of hyaline material that is quite dense optically and is referred to as the basement membrane; it can also be found underneath the endocervical surface epithelium and glands (see Chap. 2). The significance of the basement membrane in determining invasion of a cancer is discussed in Chapters 11 and 12.
Beneath the basement membrane, there is a connective tissue stroma, containing variable numbers of T and B lymphocytes, with the highest concentration in the transformation
zone (Edwards and Morris, 1985). Small, fingerlike, blood vessel-bearing projections of connective tissue (papillae) supply the epithelium with nutrients.
zone (Edwards and Morris, 1985). Small, fingerlike, blood vessel-bearing projections of connective tissue (papillae) supply the epithelium with nutrients.
Electron Microscopy
Transmission electron microscopy discloses a multilayer epithelium with cells bound to each other by numerous desmosomes. The cytoplasm is rich in glycogen and tonofibrils (see Fig. 8-3). In the most superficial epithelial layers, breakage of desmosomes is evident (Fig. 8-4) and accounts for spontaneous shedding of the superficial cells.
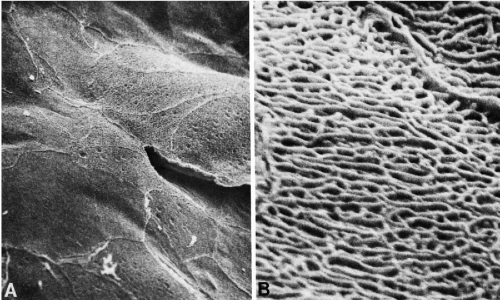
Scanning electron microscopy of the surface of the normal squamous epithelium discloses platelike arrangement of large squamous cells closely fitting with each other (Ferenczy and Richart, 1974). The surface of the cells is provided with a network of short uniform microridges. At the points of cell junctions, more prominent ridges may be noted (Fig. 8-5).
Endocervical Epithelium
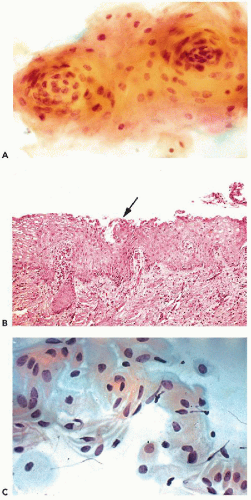
The epithelial lining of the endocervical canal, and of the endocervical glands, is formed by a single layer of mucus-producing tall columnar cells with oval nuclei and clear cytoplasm, also known as picket cells (Fig. 8-6). The endocervical epithelium participates in the events of the menstrual cycle, described below, and this is reflected by the consistency of the endocervical mucus. During the preovulatory phase of the cycle, the mucus is thick and readily crystallized; it becomes liquid just before and during the ovulation, presumably to facilitate the entry of spermatozoa into the uterine cavity. Consequently, the appearance of the cytoplasm of the endocervical cells and the position of nuclei depends on the phase of the menstrual cycle. During the proliferative phase, the cytoplasm is opaque and the nuclei are centrally located (Fig. 8-6A). During the secretory phase, the transparent cytoplasm is bulging with accumulated mucus that pushes the flattened nuclei to the basal periphery of the cells (Fig. 8-6B). In such cells, the luminal surface is flat but may show tiny droplets or smudges, reflecting secretion of mucus. The nuclei of the normal endocervical cells are open (vesicular) and spherical, and measure approximately 8 μm in diameter. Ciliated cells are commonly present in the upper (proximal) segment of the endocervical canal, as confirmed in a careful study by Babkowski et al (1996). The nuclei of the ciliated cells are somewhat larger than those of nonciliated cells (see Figs. 8-19B and 8-20D). Located among the columnar cells at the base of the epithelium, adjacent to the basement membrane, there are small, triangular basal, or reserve, cells. These cells are very difficult to see in light microscopy of normal epithelium but have been clearly demonstrated by electron microscopy. Under abnormal circumstances, a hyperplasia of the reserve cells may be observed. The role of reserve cells as the cell of origin of squamous metaplasia of the endocervix is discussed in Chapter 10.
The endocervical glands are of the simple tubular branching type, and they may vary substantially in number and distribution. In some women, the normal glands may be situated deeply within the wall of the cervix, at a considerable
distance from the surface; this distribution of endocervical glands is of importance in the diagnosis of extremely well-differentiated endocervical adenocarcinoma (see Chap. 12). The presence of glands underneath the squamous epithelium of the portio, in the area of the external os (transformation zone), is normal. The epithelium lining the glands is identical to the surface epithelium.
distance from the surface; this distribution of endocervical glands is of importance in the diagnosis of extremely well-differentiated endocervical adenocarcinoma (see Chap. 12). The presence of glands underneath the squamous epithelium of the portio, in the area of the external os (transformation zone), is normal. The epithelium lining the glands is identical to the surface epithelium.
Electron Microscopy
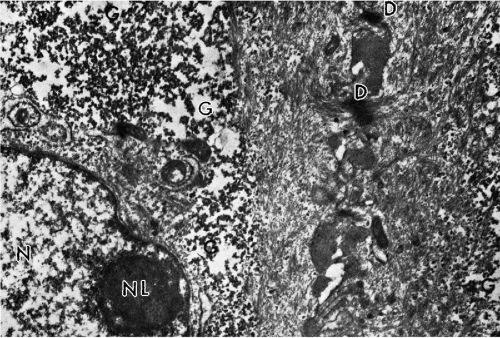
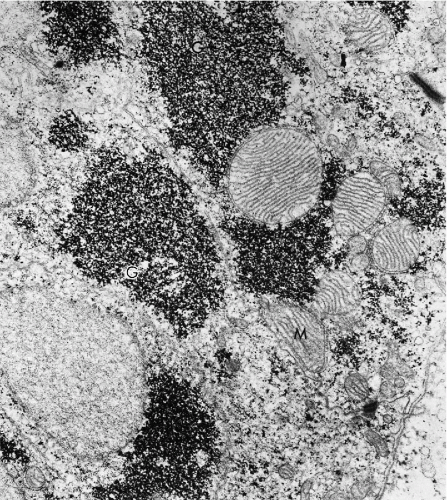
Transmission electron microscopic studies of the endocervical epithelium reveal typical, mucus-secreting cells with secretory granules in the cytoplasm. On the luminal surface, the cells are bound to each other by junctional complexes and, elsewhere, by desmosomes (Fig. 8-7). The basal reserve cells are readily observed at the base of the columnar endocervical cells.
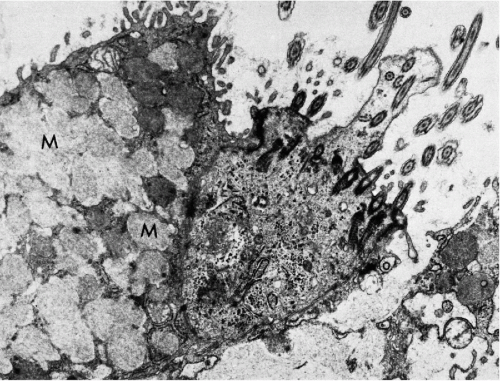 Figure 8-7 Electron micrograph of endocervical epithelium. At left there is a mucus-secreting cell, characterized by a large number of cytoplasmic granules (M); at right a ciliated epithelial cell is seen (see Fig. 8-8). (×13,000.) (Photo by Dr. H. Dembitzer, Montefiore Hospital and Medical Center, New York, NY.) |
Scanning electron microscopy shows that ciliated endocervical cells are more common than is generally estimated by light microscopy (Fig. 8-8).
Transformation Zone or the Squamocolumnar Junction
The area of the junction between the squamous and the endocervical epithelium is of considerable importance in
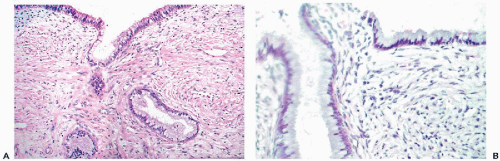
the genesis of carcinoma of the uterine cervix (see Chap. 11). In a normal, quiescent cervix, the transition between the two epithelial types is often sharp and is known as the squamocolumnar junction, now usually designated as the transformation zone (Fig. 8-9). The term transformation zone is based on colposcopic observations of adolescent and young women, documenting that the glandular epithelium of the cervix in the area of the squamocolumnar junction is undergoing constant metaplastic transformation into squamous epithelium. The events of transformation are sometimes reflected in cervical smears, showing side by side endocervical glandular cells and young metaplastic squamous cells.
the genesis of carcinoma of the uterine cervix (see Chap. 11). In a normal, quiescent cervix, the transition between the two epithelial types is often sharp and is known as the squamocolumnar junction, now usually designated as the transformation zone (Fig. 8-9). The term transformation zone is based on colposcopic observations of adolescent and young women, documenting that the glandular epithelium of the cervix in the area of the squamocolumnar junction is undergoing constant metaplastic transformation into squamous epithelium. The events of transformation are sometimes reflected in cervical smears, showing side by side endocervical glandular cells and young metaplastic squamous cells.
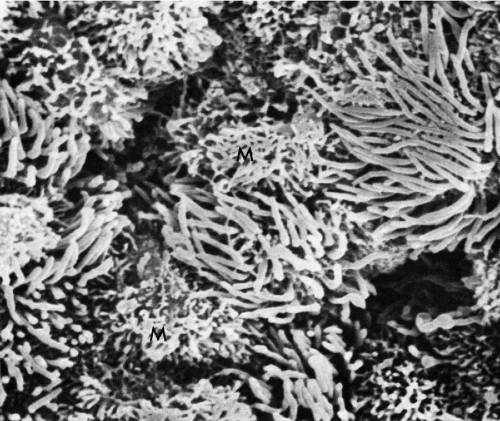 Figure 8-8 Scanning electron micrograph of endocervical epithelium. Numerous ciliated cells are next to mucus-secreting cells (M). The latter are characterized by a shaggy configuration of the surface (see Fig. 8-7). (× 4,800.) (Courtesy of Dr. Ralph Richart, New York, NY.) |
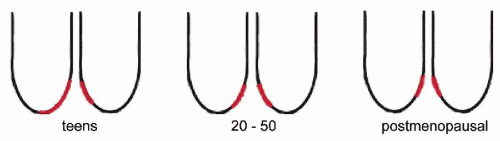
The anatomic location of the transformation zone varies considerably and is age-dependent (Fig. 8-10). In adolescents and young women, the junction is usually located at the level of the external os, but may extend to the adjacent vaginal aspect of the uterine cervix. In the latter case, the area occupied by the endocervical epithelium on the surface of the cervix may be visible to the naked eye as a sharply demarcated red area, sometimes inappropriately called an erosion, but better designated as eversion, ectropion, or ectopy. The redness reflects the presence of blood vessels under the thin endocervical epithelium. The ectropion is a benign, self-healing condition, which, however, may mimic important lesions of the cervix. The cytologic presentation and clinical significance of the ectropion are discussed in Chapter 9.
With advancing age, the junction tends to move up into the endocervical canal. At the time of the menopause, the junction is usually located within the endocervical canal and is hidden from view.
Because most of the initial precancerous changes in the uterine cervix occur within the transformation zone, this is an area of major importance in cervix cancer prevention (see Chap. 11). For this reason, much emphasis has been placed on sampling of the transformation zone by cervicovaginal smears (see comments on smear adequacy at the end of this chapter). It is evident that the transformation zone is more readily accessible in younger than in older women. For comments on cytology of the transformation zone, see below.
The Endometrium
The transition between the endocervical epithelium and the endometrium usually occurs at the level of the internal os. The transition between the large picket cells of the endocervical mucosa and the smaller cells of the endometrium is usually quite sharp.
The endometrium is essentially composed of layers of surface epithelium composed of cuboidal cells, forming simple tubular glands, surrounded by stromal cells. During the childbearing age, the endometrium undergoes cyclic changes (menstrual cycle) to prepare it for the implantation of the fertilized ovum, hence for pregnancy. The appearance of the glands and the stroma changes with the phase of the cycle, as described below. If the implantation
does not occur, the endometrium is shed before the beginning of the next menstrual cycle. A detailed history of the cyclic changes and their hormonal background can be obtained elsewhere; for our purpose, only a brief summary is necessary.
does not occur, the endometrium is shed before the beginning of the next menstrual cycle. A detailed history of the cyclic changes and their hormonal background can be obtained elsewhere; for our purpose, only a brief summary is necessary.
The Endometrium During the Menstrual Cycle
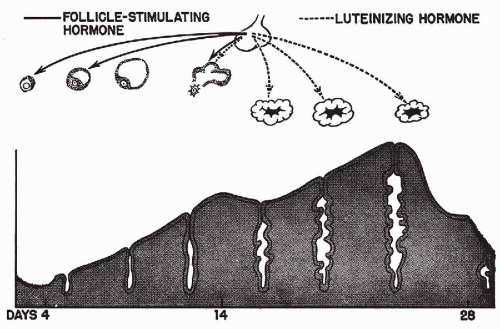
The menstrual cycle is the result of a sequence of hormonal influences that, in a normal woman, follow each other with great regularity from puberty to menopause, except during pregnancy. It has been shown by Frisch and McArthur (1974) that a certain minimal body weight in relation to height is necessary for the onset and maintenance of the menstrual activity. The ovarian hormones most directly responsible for the menstrual cycle are estrogen, produced by follicles that harbor ova, and progesterone, produced by corpus luteum that forms after expulsion of the ovum. The ovarian activity is regulated by hormones produced by the anterior lobe of the pituitary and the hypothalamus. A simple diagram summarizes the principal hormonal factors and their influence on the endometrium (Fig. 8-11).
Menstrual Bleeding
The beginning of the menstrual flow marks the first day of the cycle. It corresponds to disintegration and necrosis of the superficial portion of the endometrium, indicating
the end of the activity of progestational hormones originating in the ovarian corpus luteum. The casting off of the endometrium usually takes 3 to 5 days and is accompanied by bleeding from the ruptured endometrial vessels.
the end of the activity of progestational hormones originating in the ovarian corpus luteum. The casting off of the endometrium usually takes 3 to 5 days and is accompanied by bleeding from the ruptured endometrial vessels.
Proliferative Phase
Endometrial necrosis is followed by regeneration and the onset of the growth or proliferative phase, during which the endometrium grows in thickness. This phase of endometrial growth is under the influence of estrogens originating in the granulosa and the theca cells of the ovarian follicles and, in essence, is a preparation for pregnancy. The initial event is the regeneration of the surface epithelium from residual endometrial glands. During this stage, the endometrial surface epithelium is composed of cuboidal to columnar cells with scanty cytoplasm and spherical, intensely stained nuclei that show significant mitotic activity. Occasionally, larger cells with clear cytoplasm (helle Zellen of the Germans) are also present. Their significance is unknown.
The glands of the proliferative phase are formed by invagination of the surface epithelium. The glands are straight tubular structures lined by one or two layers of cuboidal, sometimes columnar, cells with scanty cytoplasm and intensely staining nuclei that show intense mitotic activity. The endometrial stroma in this stage is compact and formed by small cells (Fig. 8-12A). Single ciliated cells may be observed in proliferative endometrium, mainly on the surface.
Ovulation and the Secretory Phase
The release of the ovum from the ovarian follicle (ovulation) usually occurs between the 11th and 14th days of a 28-day
menstrual cycle and signals the onset of the secretory phase. The ovarian corpus luteum, which replaces the follicle, begins to function by secreting progesterone, which stimulates the secretory activity of the cells lining the endometrial glands. Secretory vacuoles, composed mainly of glycogen, are formed, at first in subnuclear position, later shifting to a supranuclear one, closer to the lumen of the gland. At the same time, the straight tubular glands become more tortuous, and the surrounding stromal cells become larger and eosinophilic, resembling decidual cells (Fig. 8-12B). There is evidence that the actual process of secretion is of the apocrine type; that is, the apical portions of the glandular cells containing glycoproteins are cast off into the lumen of the gland. With the passage of time, the tortuosity of the glands and the vacuolization of the lining cells continue to increase and the stroma becomes loosely structured. Just before the beginning of the next menstrual flow, the glands acquire a see-saw appearance before collapsing, signaling the onset of the epithelial necrosis and the beginning of a new cycle.
menstrual cycle and signals the onset of the secretory phase. The ovarian corpus luteum, which replaces the follicle, begins to function by secreting progesterone, which stimulates the secretory activity of the cells lining the endometrial glands. Secretory vacuoles, composed mainly of glycogen, are formed, at first in subnuclear position, later shifting to a supranuclear one, closer to the lumen of the gland. At the same time, the straight tubular glands become more tortuous, and the surrounding stromal cells become larger and eosinophilic, resembling decidual cells (Fig. 8-12B). There is evidence that the actual process of secretion is of the apocrine type; that is, the apical portions of the glandular cells containing glycoproteins are cast off into the lumen of the gland. With the passage of time, the tortuosity of the glands and the vacuolization of the lining cells continue to increase and the stroma becomes loosely structured. Just before the beginning of the next menstrual flow, the glands acquire a see-saw appearance before collapsing, signaling the onset of the epithelial necrosis and the beginning of a new cycle.
Electron Microscopy
Transmission electron microscopic studies of human endometrium in various phases of the cycle were carried out by several investigators. In the proliferative phase, the glands are composed of columnar cells, some ciliated, resting on a basement membrane. These cells have no distinguishing features (Fig. 8-13). The secretory phase is accompanied by a rapid formation of deposits of glycogen, which is the chief product of the glandular cells. Accumulation of glycogen and glycoproteins in the secretory phase is accompanied by formation of large mitochondria with peculiar cristae arranged in parallel fashion (Fig. 8-14) (Gompel, 1962, 1964). Scanning electron microscopic studies disclosed some differences between the epithelium of the endometrial surface and that of the endometrial glands. The endometrial surface epithelium shows few cyclic changes. The cells produce cilia and show relatively little secretory activity during the secretory part of the cycle. The epithelium lining the endometrial glands during the proliferative phase shows an intense production of cilia and microvilli. During the secretory
phase, the formation of cilia is inhibited, and, under the influence of progesterone, there is conversion of the glandular cells to the secretory function (Ferenczy, 1976; Ferenczy and Richart, 1973).
phase, the formation of cilia is inhibited, and, under the influence of progesterone, there is conversion of the glandular cells to the secretory function (Ferenczy, 1976; Ferenczy and Richart, 1973).
NORMAL CYTOLOGY OF THE UTERUS DURING CHILDBEARING AGE
Cells Originating from Normal Squamous Epithelium
Superficial Squamous Cells
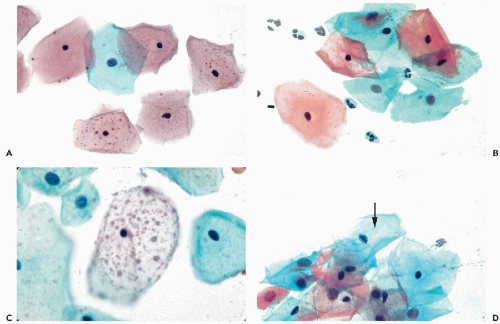
During the childbearing age of a normal woman, the bulk of cells observed in cervicovaginal smears originate from the superficial zone of mature squamous epithelium. Although several varieties of cells may originate from the surface of the squamous epithelium, the term superficial squamous cells is reserved for large polygonal cells possessing a flat, delicate, transparent cytoplasm and small, dark nuclei, averaging about 4 μm in diameter (Figs. 8-15A,B). The diameter of the superficial squamous cells is approximately 35 to 45 μm but somewhat smaller, or more often, larger cells may occur. The polygonal configuration of these cells reflects the rigidity of the cytoplasm, caused by the presence of numerous bundles of tonofibrils (intermediate filaments) seen in transmission electron microscopy (see previous). Scanning electron microscopy emphasizes the irregular configuration of these cells (Fig. 8-16). The flat surface, provided with microridges, shows a knoblike elevation of the spherical nucleus.
In well-executed Papanicolaou stains, the cytoplasm of the majority of the superficial cells stains predominantly a
delicate pink. This staining property reflects the chemical affinity of the cytoplasm for acid dyes such as eosin; hence, the term eosinophilic, or a less frequently used term, acidophilic cytoplasm. Dryness and exposure to air tend to enhance the eosinophilic properties of cells. The cytoplasm of the superficial cells may, at times, stain a pale blue, reflecting a slight affinity for basic dyes such as hematoxylin. Intense blue staining (cyanophilia) of the cytoplasm of superficial cells should not be seen in Papanicolaou stain, although it may be seen with other staining procedures such as the Shorr’s stain (see Chap. 44).
delicate pink. This staining property reflects the chemical affinity of the cytoplasm for acid dyes such as eosin; hence, the term eosinophilic, or a less frequently used term, acidophilic cytoplasm. Dryness and exposure to air tend to enhance the eosinophilic properties of cells. The cytoplasm of the superficial cells may, at times, stain a pale blue, reflecting a slight affinity for basic dyes such as hematoxylin. Intense blue staining (cyanophilia) of the cytoplasm of superficial cells should not be seen in Papanicolaou stain, although it may be seen with other staining procedures such as the Shorr’s stain (see Chap. 44).
Small, dark brown cytoplasmic granules are often visible, usually in a perinuclear location but, occasionally, they are also present in the periphery of the cytoplasm (see Fig. 8-15A). Masin and Masin (1964) documented that the granules contain lipids and that their presence is estrogen dependent. Occasionally, larger, spherical, pale brown inclusions of variable sizes may be observed in the cytoplasm of the superficial squamous cells, which have been named polka-dot cells (Fig. 8-15C). The nature of these inclusions is unknown. Some observers consider such cells to be an expression of human papillomavirus (HPV) (summary in DeMay, 1996). In our experience, such inclusions are uncommon and occur mainly in poorly preserved or degenerated squamous cells. The polka dot cells do not correspond to any known disease state, a view also shared by Schiffer et al (2001). Superficial squamous cells with vacuolated cytoplasm, resembling fat cells, have also been considered by some as reflecting HPV infection. In our experience, such cells are usually the result of treatment by radiotherapy or cautery (see Chap. 18).
The superficial squamous cells are the end-of-the-line dead cells and this is reflected in their small nuclei, which are pyknotic, that is, the nuclear material has become condensed and shrunken. A narrow clear zone often surrounds the condensed nucleus, indicating the area occupied by the nucleoplasm before shrinkage (see Fig. 8-15A,B). Sometimes the nuclear chromatin may be fragmented and broken into small granules, suggestive of karyorrhexis and, hence, apoptosis (see Chap. 6). Upon close inspection of such cells, minute detached fragments of nuclear material may be seen in the vicinity of the main nuclear mass. In phase microscopy, the pyknotic nuclei display a characteristic reddish hue.
Since complete maturity of the epithelium can rarely occur in the absence of estrogens, nuclear pyknosis in mature
superficial cells constitutes morphologic evidence of estrogenic activity. This feature is of value in the analysis of hormonal status of the patient (see Chap. 9).
superficial cells constitutes morphologic evidence of estrogenic activity. This feature is of value in the analysis of hormonal status of the patient (see Chap. 9).
Intermediate Squamous Cells
The intermediate-type cells are of the same size as the superficial cells or somewhat smaller. Their cytoplasm is usually basophilic (cyanophilic) and occasionally somewhat more opaque in the Papanicolaou stain, although eosinophilic cells of this type may occur. The chief difference between the superficial and the intermediate cells lies in the structure of the nucleus; the nuclei of the intermediate cells measure about 8 μm in average diameter, are spherical or oval, with a clearly defined nuclear membrane surrounding a well-preserved homogeneous, faintly granular nucleoplasm. Chromocenters and sex chromatin may be observed within such nuclei. The term vesicular nuclei is applied to define this type of nuclear configuration.
It is not uncommon to observe in the nuclei of normal intermediate cells nuclear grooves or creases in the form of straight or branching dark lines (review in Payandeh and Koss, 2003). In some cases, chromatin bars with short lateral extensions (caterpillar nuclei), are observed along the longer axis of oval nuclei (Fig. 8-15D). Such bars are commonly observed in the nuclei of squamous cells in oral and conjunctival smears, discussed in Chapters 21 and 41. Kaneko et al (1998) suggested that the nuclear creases or bars represent an infolding of the nuclear membrane but the mechanism of their formation remains unknown. It has been documented that the presence or frequency of nuclear grooves is not related to either inflammatory or neoplastic events (Payandeh and Koss, 2003).
A variant of the intermediate cells is the boat-shaped navicular cell (from Latin, navis = boat). These approximately oval-shaped cells store glycogen in the form of cytoplasmic deposits that stain yellow in Papanicolaou stain, and push the nucleus to the periphery (see Figs. 8-27B and 8-31A). The navicular cells are commonly seen in pregnancy and may be observed in early menopause (see below).
It must be emphasized that, under a variety of physiologic and pathologic circumstances (pregnancy, certain types of menopause, hormonal deficiencies, inflammation), the squamous epithelium of the female genital tract may fail to reach full maturity. In such cases, the intermediate, or sometimes even parabasal cells, form the epithelial surface and become the preponderant cell population in smears (see below and Chap. 9).
Physiologic Variations of the Superficial and Intermediate Squamous Cells
Cytoplasmic folding, often accompanied by clumping of cells is a normal phenomenon occurring during the last third of the menstrual cycle, prior to the onset of menstrual bleeding. Cytoplasmic folding may also occur during pregnancy (see below). Folding and clumping are often accompanied by lysis of the cytoplasm (cytolysis) caused by lactobacilli (see below; see also Fig. 8-31B).
The superficial and intermediate cells may form tight whorls or “pearls” in which the cells are concentrically arranged, in an onion-like fashion (Fig. 8-17A,B). The
whorls are often interpreted as reflecting estrogenic effect, but the proof of this is lacking. This must be differentiated from a similar arrangement of cells with abnormal nuclei, occurring in squamous carcinoma (see Chap. 11). An elongation of the intermediate cells, resulting in a spindly shape, has been observed at times (Fig. 8-17C). Such cells may somewhat resemble smooth-muscle cells (see Fig. 8-36). The identification of spindly squamous cells is facilitated in the presence of transitional forms of these cells, as shown in Figure 8-17C. Benign spindly squamous cells must also be differentiated from similarly shaped cancer cells with abnormal nuclei (see Chap. 11).
whorls are often interpreted as reflecting estrogenic effect, but the proof of this is lacking. This must be differentiated from a similar arrangement of cells with abnormal nuclei, occurring in squamous carcinoma (see Chap. 11). An elongation of the intermediate cells, resulting in a spindly shape, has been observed at times (Fig. 8-17C). Such cells may somewhat resemble smooth-muscle cells (see Fig. 8-36). The identification of spindly squamous cells is facilitated in the presence of transitional forms of these cells, as shown in Figure 8-17C. Benign spindly squamous cells must also be differentiated from similarly shaped cancer cells with abnormal nuclei (see Chap. 11).
Parabasal Cells
The parabasal squamous cells vary in size and measure from 12 to 30 μm in diameter. The nuclei are vesicular in type and similar to the nuclei of intermediate squamous cells. The frequency of occurrence and the morphologic presentation of parabasal squamous cells in cervicovaginal smears depend on the technique of securing the sample.
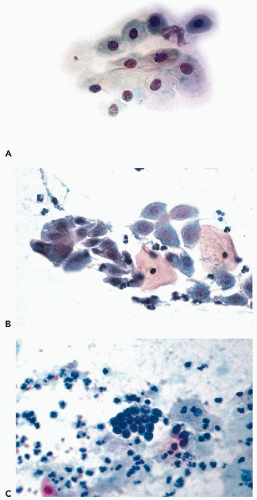
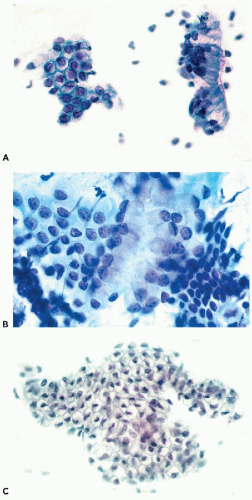
In vaginal pool smears obtained by a pipette or a blunt instrument, spontaneously exfoliated parabasal cells occur singly and are usually round or oval in shape, with smooth cytoplasmic borders (Fig. 8-18A). The cytoplasm is commonly basophilic (cyanophilic) and occasionally contains small vacuoles. Exposure to air and dryness may cause cytoplasmic eosinophilia. The nuclei are usually bland and homogeneous. This appearance of parabasal cells results from contraction of the cytoplasm following cell death and breakage of desmosomes that occurred prior to desquamation. Few cells of this type are seen in normal smears from women in their 20s and early 30s, but the number increases in women more than 35 years of age. Such cells may become the dominant cell type in postmenopausal women with epithelial atrophy (see below). In the presence of inflammatory processes within the vagina or the cervix with resulting damage to the superficial and intermediate layers of the squamous epithelium, the proportion of parabasal cells in smears may increase substantially (see Chap. 10).
In direct cervical scrapes and brush smears, the proportion of parabasal cells is much higher than in vaginal pool smears. Such cells are derived from areas of immature squamous epithelium and areas of squamous metaplasia of the endocervical epithelium in the transformation zone and the endocervical canal. For further discussion of squamous metaplasia (see Chap. 10). In cervical scrape smears, such cells are trapped in streaks of endocervical mucus. In preparations obtained by endocervical brushes and in preparations obtained from liquid fixatives, the relationship of parabasal cells to endocervical mucus is lost.
Parabasal cells forcibly dislodged from their epithelial setting by an instrument are often angular and have irregular polygonal shapes. Such cells occur singly, but often form flat clusters that vary in size from a few to several hundred cells. In clusters, such cells often form a mosaic-like pattern, in which the contours of the cells fit each other (Figs. 8-9D and 8-18B). The term metaplastic cells is often used to describe such cells, although their origin from squamous metaplasia is not always evident or secure. The reason for the angulated appearance of parabasal cells is the presence of intact desmosomes that bind the adjacent cells together. As the cytoplasm shrinks during the fixation process, the desmosomes are not affected and, consequently, the portions of the cytoplasm attached to the desmosomes stretch and become elongated, giving the cells an angulated appearance (see Fig. 8-18B). Thus, the angulated appearance of the parabasal cells of “metaplastic” type, whether occurring singly or in clusters, is a useful fixation artifact.
The nuclei of parabasal cells, which measure about 8 μm in diameter, show a fine network of chromatin, chromocenters, and, occasionally, very small nucleoli. When compared with superficial or intermediate cells, the nuclei of parabasal cells occupy a much larger portion of the total cell volume and, therefore, give the erroneous impression of being larger. I have not observed mitotic figures among normal parabasal cells in smears.
The presence of parabasal cells in smears is of interest in defining an “adequate cervical smear,” which is often judged by the presence of “metaplastic” cells derived from the transformation zone and the endocervical canal (for further discussion of smear adequacy, see end of this chapter). It is evident that when the transformation zone is readily accessible to sampling, as in women of childbearing age, it will be better represented in the smears than in older women (see Figs. 8-9D and 8-10).
Basal Cells
Because of their protected status, the basal cells are practically never seen in smears. If present, it may be safely assumed that a pathologic process or vigorous brushing has damaged the upper layers of the squamous epithelium, resulting in the appearance of these very small round or oval cells, resembling miniature parabasal cells. Their very scanty cytoplasm is basophilic but may become eosinophilic in dry smears (Fig. 8-18C). The nuclei are of the same size as those of the parabasal cells but, because of the small size of the cells, appear to be larger. The nuclei display fine chromatin structure with chromatin granules and, occasionally, tiny round nucleoli. The uncommon normal basal squamous cells should not be confused with small cancer cells that may be of similar size and configuration (see Chap. 11).
Dendritic Cells and Langerhans Cells
These cells have never been identified by us in normal smears, although their presence in the histologic sections of the squamous epithelium has been well documented, as previously described.
Cells Originating from the Endocervical Epithelium
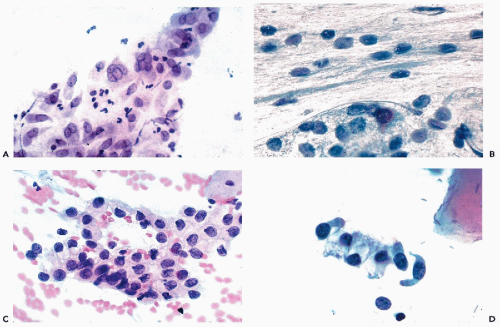
In vaginal pool smears, the endocervical cells are relatively uncommon and rarely well preserved. In cervical smears obtained by means of instruments, particularly endocervical brushes, the endocervical cells are usually numerous and well preserved. When seen in profile, the endocervical cells are columnar and measure approximately 20 μm in length and from 8 to 12 μm in width (Fig. 8-19A). Shorter cells, of plump, more cuboidal configuration may also occur. The columnar endocervical cells may occur singly but, quite often, they are seen as sheets of parallel cells, arranged in a palisade (Fig. 8-19B). When the endocervical cells are flattened on the slide and are seen “on end,” they form tight clusters or plaques, wherein the cells form a tightly fitting mosaic resembling a honeycomb. In such plaques, the cell membranes form the partitions of the honeycomb and the centers are filled by clear cytoplasm surrounding the nuclei (Fig. 8-19A,C). The identification of such cells as endocervical is facilitated if columnar cells are present at the periphery of the cluster.
The cytoplasm of endocervical cells is either finely vacuolated or homogeneous and faintly basophilic or distended by clear, transparent mucus that is pushing the nuclei toward
the narrow end of the cell. Some such cells may become nearly spherical in shape because of cytoplasmic distention by mucus. On the surface of the mucus-containing cells, small droplets or smudges of mucus may be observed.
the narrow end of the cell. Some such cells may become nearly spherical in shape because of cytoplasmic distention by mucus. On the surface of the mucus-containing cells, small droplets or smudges of mucus may be observed.
The nuclei are spherical or oval, vesicular in configuration, with delicate chromatin filaments, often showing chromocenters and very small nucleoli. The nuclei may vary in size. The dominant size of the nuclei is about 8 μm in diameter but larger nuclei, up to 15 or 16 μm in diameter, are not uncommon. The variability of the nuclear sizes may reflect stages in cell cycle or other, unknown factors. Multinucleated cells may also occur (Fig. 8-20A). The fragile cytoplasm of the endocervical cells may disintegrate, with resulting stripped, or naked, nuclei, usually of spherical or somewhat elliptical configuration (Fig. 8-20B). These nuclei may also vary in size and may be difficult to recognize, unless they are similar to, or identical with, the nuclei of adjacent better-preserved endocervical cells. Small intranuclear cytoplasmic inclusions in the form of clear areas within the nucleus may occur in endocervical cells (Fig. 8-20B).
At the time of ovulation, and sometimes during the secretory (postovulatory) phase of the menstrual cycle, the nuclei of endocervical cells form intensely stained, dark, nipplelike protrusions of various sizes, up to 3 μm in length, that are an extension of the nucleus into the adjacent cytoplasm (see Figs. 8-19C and 8-20C). The protrusions appear mainly on the lumenal aspect of the nucleus, facing the endocervical lumen. Sometimes the protrusions are split in two. All stages of formation of the protrusions may be observed, ranging from a thickening of the nuclear membrane to protrusions growing in size. In nuclei with fully developed protrusions, the remainder of the nucleus is usually less dense and transparent, suggesting that there has been a shift of the chromatin to the protrusion. The mechanism of formation and the nature of the protrusions are the subject of a considerable debate. Taylor (1984) thought that the protrusions occurred mainly in ciliated endocervical cells and that their formation was the result of high ciliary activity. McCollum (1988) observed the protrusions in women receiving the long-term contraceptive drug medroxyprogesterone, during periods of amenorrhea, when the estrogenic activity was low. McCollum thought that the protrusions represented an attempt at nuclear division arrested by progesterone and, therefore, consistent with events occurring at the onset of ovulation. Zaharopoulos et al (1998) studied the protrusions by a number of methods, including electron microscopy, cytochemistry, and in situ hybridization of X chromosome. These investigators observed the presence of nucleoli and single X chromosome within the protrusions and reported findings suggestive of formation of an abortive mitotic spindle attached to the protrusion, thus providing support to McCollum’s suggestion that the protrusion represents an attempt at mitotic division. Although further studies may shed some additional light on this very interesting phenomenon, it is quite certain that the protrusions do not represent an artifact, as has been suggested by Koizumi (1996). It is of note that similar protrusions may be occasionally observed in histologic sections of the endocervix during the secretory phase of the cycle and in epithelial cells of various origins, for example, in bronchial epithelial cells and in duct cells of the breast obtained by aspiration (see Chaps. 19 and 29). Zacharopoulos et al observed similar nuclear protrusions in occasional nonepithelial cells, suggesting that the phenomenon
is of a general nature and clearly worthy of further studies.
is of a general nature and clearly worthy of further studies.
Ciliated Endocervical Cells
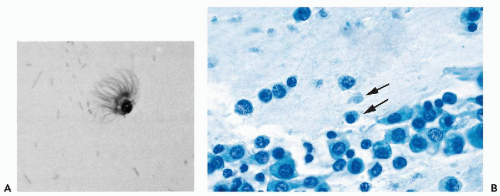
Endocervical cells showing recognizable cilia, supported by a terminal plate, are fairly frequent, particularly in brush specimens from the upper (proximal) segments of the endocervical canal. The nuclei of such cells are sometimes larger than average and somewhat hyperchromatic (Fig. 8-20D). The presence of the ciliated cells has been interpreted by some as evidence of tubal metaplasia, an entity that is discussed in Chapter 10.
Hollander and Gupta (1974) were the first to report the presence of detached ciliary tufts in cervicovaginal smears (Fig. 8-21A). This very rare event, occurring in about onetenth of 1 percent of smears, cannot be correlated with time of cycle or age of patients. The ciliary tufts are fragments of ciliated endocervical cells, although sometimes their origin from the endometrium, or even the fallopian tubes, cannot be excluded. Next to detached ciliary tufts, remnants of the cell body with pyknotic nuclei may sometimes be observed (Fig. 8-21B). The phenomenon is similar to ciliocytophthoria, which was described by Papanicolaou in ciliated cells from the respiratory tract (see Chap. 19). So far, there is no evidence that the detached ciliary tufts in cervicovaginal smears are related to a viral infection, which may be the cause of ciliocytophthoria in the respiratory tract, and the mechanism of their formation is not clear.
The tiny basal cells of the endocervical epithelium have never been identified by us with certainty in normal smears although, undoubtedly, they should occur in energetic endocervical brush specimens.
Endocervical Cells and the Menstrual Cycle
The changes in the consistency of the cervical mucus during the menstrual cycle were mentioned above and will be discussed again below in the assessment of ovulation in Chapter 9. It was suggested by Affandi et al (1985) that the morphology of the endocervical cells follows the events in the cell cycle. In the proliferative (preovulatory) phase, the cytoplasm of the endocervical cells in sheets is opaque and scanty and the nuclei are closely packed together. In the secretory (postovulatory) phase of the cycle, the cytoplasm is distended with clear mucus, the nuclei show degeneration (which, to this writer, appear to reflect the “nipple” formation described above), and, in cell sheets, are separated from each other by areas of clear cytoplasm. Affandi et al suggested that these differences in endocervical cell morphology in smears may be used to determine the occurrence of ovulation as reliably as endometrial biopsies. Affandi’s observations have not been tested (see Chap. 9).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree