and Andrew B. Williams2
(1)
Department of Colorectal Surgery, Guy’s and St. Thomas’s Hospitals NHS Foundation Trust, London, UK
(2)
Department of General Surgery, Guy’s and St. Thomas’ Hospitals NHS Foundation Trust, London, UK
Abstract
A comprehensive understanding and assessment of the anal canal play a vital role in the correct diagnosis and management of patients with anal canal disorders. Imaging of the anal canal may be performed using either AES or MRI, for evaluation of anal cancer, anal fistula and anal incontinence. Staging of early anal cancer is superior with AES though MRI is superior in the assessment of distant lymph node metastasis. MRI is slightly superior in the assessment of anal sepsis although AES does provide a useful alternative. AES is the gold standard in assessing anal sphincter integrity following obstetric injury. A wide variety of tests are available to assess the physiology of the anal canal. Physiological assessment gives an objective measure in patients with both faecal incontinence and difficult defaecation. The understanding of the anal canal anatomy and physiology is vital in the correct management of patients with anal disorders.
Anal Canal Anatomy and Histology
The anal canal begins at the anorectal junction as the rectum passes through the pelvic cavity at the cranial end of the levator ani muscle forming a sharp angle. The anal canal in adults is between 2.5 and 5 cm in length extending caudally to terminate at the anal verge [1].
The anal canal is derived from the caudal hindgut which is divided by the urorectal (cloacal) septum into the urogenital sinus (giving rise to the urethra and bladder) and rectum [2]. The rectum and superior anal canal are separated from the outside by the anal membrane which breaks down by the end of the eighth week of gestation. Abnormal separation of the cloaca, with the anal canal posteriorly and the urethra and bladder anteriorly, results in a variety of anorectal malformations. An abnormality in the development of the urorectal septum in a posterior direction leads to the majority of the anorectal abnormalities such as rectal atresia and fistulas between the rectum and urethra, urinary bladder or vagina.
The anal canal epithelium provides the transition point between the proximal rectal intestinal columnar epithelium to the distal stratified epithelium of the skin.
The rectum has a thick muscularis propria layer, containing an inner circular and outer longitudinal muscle layer of rectal muscle, which is capable of peristaltic activity. The muscularis mucosa is also a prominent feature of the rectum producing a rhythmic contraction which prevents clogging of the glands and enhances expulsion of mucus. The rectal epithelium has numerous mucus-secreting goblet cells that lubricate the stool and absorptive cells to absorb water from the stool which are arranged in closely packed tubular glands, greatly increasing surface area.
The anal canal epithelium is divided in to three sections. The proximal canal (cranial end of puborectalis to the cranial end of the dentate line) is lined by simple columnar epithelium. The epithelium in the proximal section is similar to that of the rectum but has shorter more irregular crypts and more smooth muscle fibres in the lamina propria layer. This changes to stratified squamous epithelium lower in the anal canal along an intermediate transition zone just above the dentate line [3]. The transition zone epithelium (zone between uninterrupted columnar mucosa above and uninterrupted squamous epithelium below) has a variable length of between 3 and 11 mm. In the fetus and newborn, there is a sharp transition, but in the adult this transition is not so sharp and stratified squamous epithelium may be present on columns. The transitional zone epithelium produces minimal mucin and may have features of squamous epithelium but contains anal glands and endocrine cells in the submucosal layer. The distal zone (caudal end of the dentate line to the anal verge) contains non-keratinizing squamous epithelium. The epithelium at this level does not contain glands or hair follicles. The squamous epithelium of the anal canal merges with perianal skin (with keratin, hair follicles and apocrine glands) at anal verge.
The dentate (pectinate) line is at the transitional zone and identified at the caudal end of the anal valves where they form to create a circumferential line. The anal mucosa forms between five and ten anal columns (columns of Morgagni) cranial to the dentate line. The columns are separated by anal valves which are easily identifiable in children but are more difficult to identify in adults [2]. The anal valves contain the rectal venous plexus. The anal glands discharge via the anal crypts at the level of the dentate line. The anal crypts are not present below the dentate line.
The layer deep to the anal mucosa is formed by subepithelial tissue, which is composed of connective tissue and smooth muscle [1]. Anal (vascular) cushions are a normal structure of anal canal, found within this subepithelial layer; these contribute to anal closure. The cushions contain blood vessels, connective tissue and smooth muscle. The anal cushions are thought to be important in the maintenance of continence and increase in thickness throughout life.
Beneath the subepithelial layer, the caudal continuation of the circular smooth muscle of the rectum thickens to form the internal anal sphincter [3]. The internal anal sphincter begins as the circular smooth muscle passes through the cranial end of puborectalis and terminates caudally in a clearly defined edge at a variable distance from the anal verge.
The continuation of the longitudinal muscle layer of the rectum forms the longitudinal muscle layer in the anal canal and lies between the internal and external anal sphincters in the intersphincteric space. The anal glands that open via the anal crypts along the dentate line are found in the intersphincteric space. The longitudinal muscle comprises smooth muscle cells from the outer layer of the rectal wall and striated muscle from levator ani, puborectalis and pubococcygeus. Caudally the fibres from this layer pass through the external anal sphincter forming septa that insert into the skin of the lower anal canal and adjacent perineum as the corrugator cutis ani muscle, giving the radial corrugation of perianal skin.
The external anal sphincter is made up of striated muscle and surrounds the longitudinal muscle forming the outer border of the intersphincteric space. Figure 1.1 shows a sagittal view of the anal canal. The widely held opinion is that the external anal sphincter is a tripartite structure [4]. In this arrangement the external sphincter is divided into deep, superficial and subcutaneous parts. The deep section of the external anal sphincter is fused with puborectalis, with the deep and subcutaneous parts of the sphincter forming rings of muscle, between them elliptical fibres from the superficial part of the external anal sphincter run anteriorly from the perineal body to the coccyx posteriorly. Other models of the external anal sphincter describe it as a single muscle continuous with the puborectalis muscle [3], or one that has a two-part structure [5]. The two-part model suggested a deep external anal sphincter and a superficial external anal sphincter. The two-part model corresponds to the puborectalis and deep external anal sphincter combined and the fused superficial and subcutaneous sphincter of the tripartite model. The external anal sphincter muscle may not be complete in certain places, and it has been suggested that the smooth and skeletal muscles form an intimately integrated anal sphincter complex.
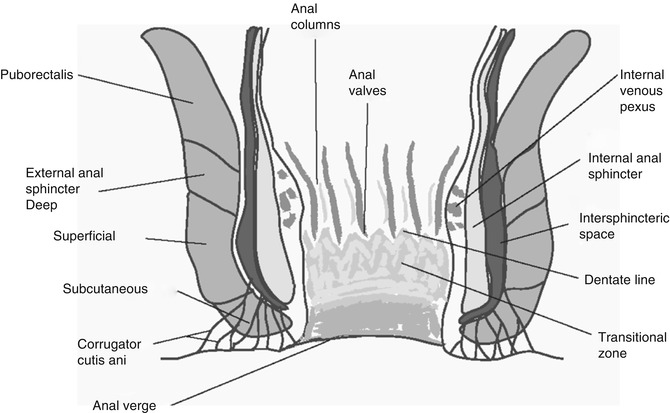

Fig. 1.1
Sagittal view through the normal anal canal
Imaging of the anal canal using anal endosonography (AES) and magnetic resonance imaging (MRI) has not completely resolved the anatomical question regarding the external anal sphincter. Anal canal imaging in women suggested that the anterior external anal sphincter is shorter than the posterior and that the lateral external anal sphincter length was longer than the anterior/posterior length, whereas a more cylindrical uniform shape was present in males. It is however more likely that the women simply have a shorter external anal sphincter than males and it does not actually differ in configuration [6].
The external anal sphincter is innervated by the pudendal nerve (S2–S4) [1], which leaves the pelvis via the lower part of the greater sciatic notch, where it passes under the lower border of the piriformis muscle. It then crosses the ischial spine and sacrospinous ligament to enter the ischioanal fossa through the lesser sciatic notch or foramen via the pudendal (or Alcock’s) canal.
The pudendal nerve has two branches: the inferior rectal nerve (mainly S2 and S3), which supplies the external anal sphincter and sensation to the perianal skin, and the perineal nerve, which innervates the anterior perineal muscles together with the sphincter urethrae and forms the dorsal nerve of the clitoris/penis. In monkeys there is a degree of cross-innervation between the two sides of the sphincter, although the degree of overlap in humans is limited. Puborectalis receives its main innervation from a direct branch of the fourth sacral nerve root; it may derive some innervation via the pudendal nerve.
The autonomic supply to the anal canal and pelvic floor comes from two sources. The fifth lumbar nerve root sends sympathetic fibres to the superior and inferior hypogastric plexuses, and the parasympathetic supply is from the second to fourth sacral nerve roots via the nervi erigentes [1]. Fibres of both systems pass obliquely across the lateral surface of the lower rectum to reach the region of the perineal body. The internal anal sphincter has an intrinsic nerve supply from the myenteric plexus together with an additional supply from both the sympathetic and parasympathetic nervous systems.
The blood supply to the anal canal cranially, in keeping with its hind gut origin, is from the superior rectal artery (branch from the inferior mesenteric artery) as its terminal branches pass from the rectum to the anal canal between the mucosa and muscle layers. These arteries anastomose with the middle rectal artery, a branch of the internal iliac artery. The inferior rectal artery is a branch from the internal pudendal artery arising from the lateral wall of the ischioanal fossa, crossing the fossa to supply the caudal anal canal muscles and skin. The inferior rectal artery anastomoses with the middle and superior rectal arteries in the anal canal.
Venous drainage of the anal canal is via the internal venous plexus in the submucosa and external plexus outside the muscular layer. The internal plexus drains mainly to the superior rectal veins and then into the inferior mesenteric veins. The external and to a lesser extent the internal plexuses drain into the inferior rectal vein which drains into the internal pudendal vein. The internal venous plexus is a site of a portocaval venous anastomosis which may lead to varices in portal hypertension.
The lymphatic drainage of the cranial anal canal is via the anorectal then perirectal lymph nodes to the preaortic lymph nodes. The lymphatic drainage to caudal anal canal is to the internal and common iliac lymph nodes. The lymphatic drainage of the skin of the anal canal is to the medial superficial inguinal lymph nodes.
Anal Canal Physiology
Anal continence is maintained by the complex interaction of many different variables. Stool must be delivered at a suitable rate from the colon into a compliant rectum of adequate volume. The consistency of this stool should be appropriate and accurately sensed by the sampling mechanism. Sphincters should be intact and able to contract adequately to produce pressures sufficient to prevent leakage of flatus, liquid and solid stool. For effective defaecation there needs to be coordinated relaxation of the striated muscle components with an increase in intra-abdominal pressure to expel the rectal contents. The structure of the anorectal region should prevent herniation or prolapse of elements of the anal canal and rectum during defaecation.
As a result of the complex interplay between the factors involved in continence and faecal evacuation, a wide range of investigations are needed for full assessment. A defect in any one element of the system in isolation is unlikely to have great functional significance, and so in most clinical situations, there is more than one contributing factor.
Anorectal physiological studies alone cannot separate the different structures of the anal canal; instead they provide measurements of the resting and squeeze pressures along the canal. Between 60 and 85 % of resting anal pressure is attributed to the action of the internal anal sphincter [7]. The external anal sphincter and the puborectalis muscle generate maximal squeeze pressure [8]. Symptoms of passive anal leakage (where the patient is unaware that episodes are happening) are attributed to internal sphincter dysfunction, whereas urge symptoms and frank incontinence of faeces are due to external sphincter problems.
The function of the internal anal sphincter is under autonomic control. The sympathetic nerve supply to the internal anal sphincter is excitatory, and the parasympathetic supply is inhibitory. Sympathetic nervous activity is thought to enhance and parasympathetic activity to reduce internal sphincter activity. Relaxation of the internal anal sphincter may be mediated via non-adrenergic, non-cholinergic nerve activity via the neural transmitter nitric oxide. The internal anal sphincter is in a state of tonic contraction; as the rectum distends, to a certain volume, there is reflex relaxation of the internal anal sphincter and followed by a contraction of the external anal sphincter (rectoanal inhibitory reflex).
The rectum, most of the time, is empty as a result of the angulation at the rectosigmoid junction. As the volume of faeces increases in the sigmoid colon, peristalsis forces faeces into the rectum. The volume required to elicit the rectoanal inhibitory reflex correlates with the volume required for first sensation [9]. As the rectal wall distends, afferent signals spread through the myenteric plexus and initiate a peristaltic wave in the descending colon, sigmoid colon and rectum. The internal anal sphincter then relaxes, due to inhibitory signals from the myenteric plexus, and contents from the rectum enter the anal canal and to come in contact with the transition zone mucosa, allowing discrimination of solid from fluid and flatus, a process vital to maintaining continence. In order to prevent incontinence there is a reflex contraction of the external anal sphincter during this anorectal sampling process. The rate of recovery of sphincter tone after this relaxation differs for the proximal and distal canal, which may be important in maintaining continence [10]. In patients with Hirschsprung’s disease, progressive systemic sclerosis, Chagas disease and initially after a coloanal anastomosis, there is no reflex relaxation of the internal anal sphincter. Further studies investigating the role of the rectoanal inhibitory reflex have shown that there is a greater sphincter relaxation of the anal sphincters in incontinent patients as rectal volume increases and patients with defaecatory difficulties have a greater recovery velocity of the resting anal pressure in the proximal anal canal [9].
The rectal defaecatory reflex described above is weak and is fortified by the parasympathetic defaecatory reflex in order to perform effective defaecation. The parasympathetic reflex involves the sacral segment of the spinal cord. The nerve endings in the rectum transmit signals to the spinal cord when stimulated. There is then a reflex stimulation to the descending colon, sigmoid colon and rectum via the parasympathetic nerve fibres in the pelvic nerves. The parasympathetic signals intensify the peristaltic wave and relax the internal anal sphincter. The combined parasympathetic and sympathetic reflexes can sometimes effectively empty the left side of the colon in one movement. In patients following spinal injury, the reflex may still be present and accounts for the involuntary evacuation seen in these patients.
Defaecation is voluntarily inhibited by contraction of puborectalis and the external anal sphincter; this allows defaecation to be deferred to a more convenient time. Voluntary defaecation is initiated by relaxing puborectalis and the external anal sphincter and contracting the abdominal muscles. Defaecation is best performed in the squat position (knees higher than hips, with the back straight and elbows on knees), allowing the anorectal angle to straighten aiding defaecation.
The gastrocolic reflex is another important reflex in the timing of defaecation. As the stomach distends, there is an initiation of rectal contraction and a desire to defaecate. The exact mechanism is uncertain but may be due to the effects of gastrin on the colon. In children this reflex is dominant and defaecation follows meals, whereas in adults cultural factors and individual habits determine when defaecation occurs.
Normal defaecatory patterns vary considerably between individuals with some opening their bowels 3 times a day and others every 3 days.
No standardisation exists for either the equipment or technique used for anal canal physiology. A variety of different systems have been used, and it is important to note that measurements differ depending on the system employed. Available systems include microballoons filled with air or water, microtransducers and water-perfused catheters. These systems may be hand-held or automated. Hand-held systems are withdrawn in a measured stepwise fashion with recordings made after each step (usually of 5–10 mm intervals), or a single measurement is recorded at the point of maximum rest or squeeze anal canal pressure. The automated systems withdraw the catheter at set rates and allow continuous data recording (usually at 1 mm intervals).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


