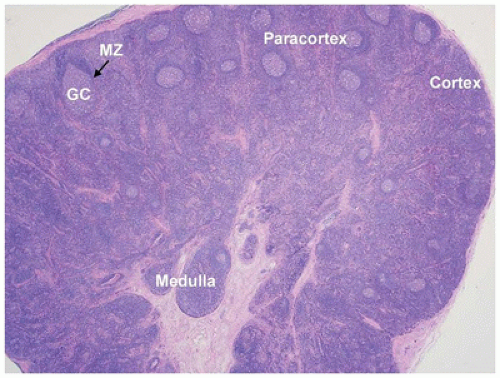
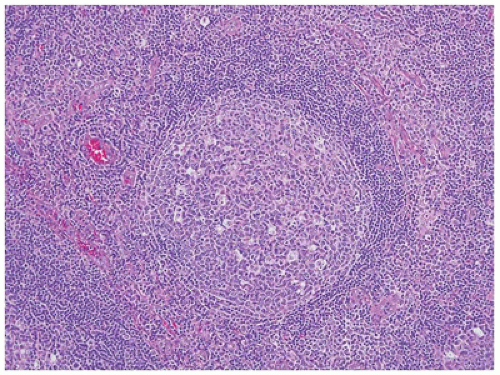
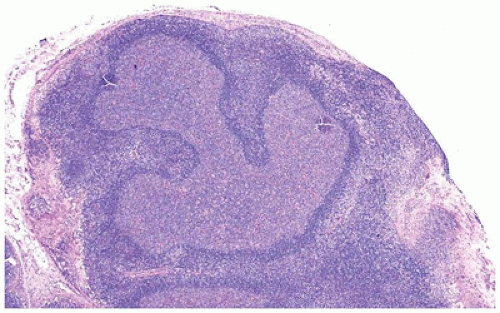
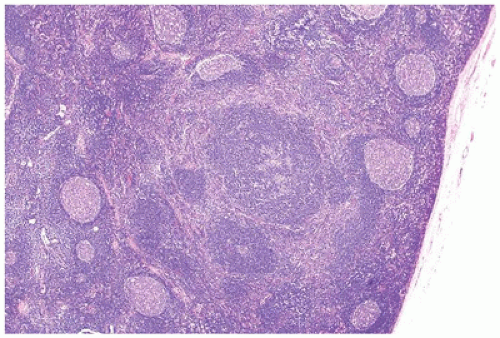
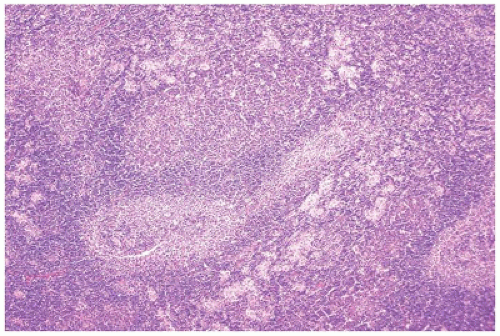
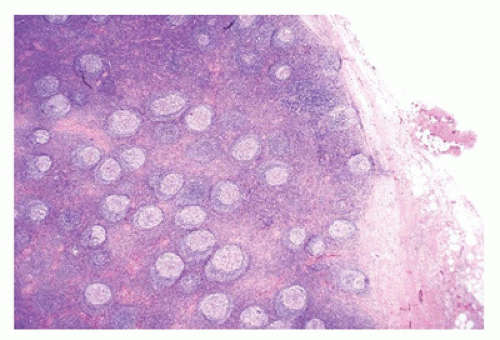
no response to antibiotic therapy or a failure to regress after 6 weeks without identification of an infectious etiology (6).
generated. In the appropriate clinical setting, some karyotypic abnormalities may be pathognomonic for certain types of malignancies (Table 23-3) and can therefore be used for diagnostic/classification purposes (8,9). In other settings, especially precursor B-cell lymphoblastic lymphoma/leukemia, the results of cytogenetic studies can also be used for prognostic purposes (10). The most common karyotypic changes related to lymphoproliferative disorders in children include translocations of immunoglobulin and T-cell receptor loci, which are frequently paired with loci involved in normal development and hematopoiesis (11,12). A major advance in diagnostic cytogenetics is the widespread availability of fluorescent in situ hybridization (FISH) studies for these translocations. Two major advantages of FISH techniques over routine cytogenetics are (a) rapid turnaround (<24 hours) and (b) ability to utilize fixed tissues including touch preparations, cytospin preparations, or sections of paraffin-embedded tissues (13,14).
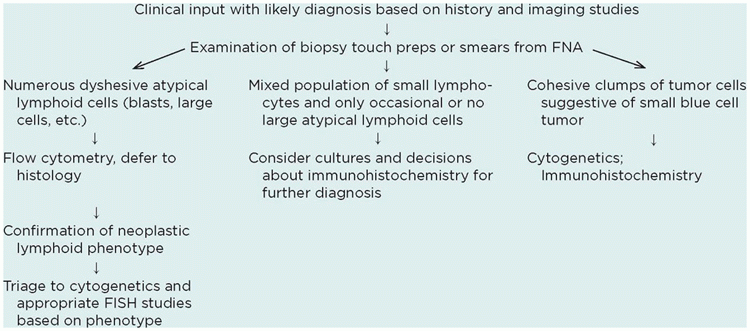
TABLE 23-1 APPROACH TO DIAGNOSIS AT THE TIME OF BIOPSY | |
|---|---|
|
TABLE 23-2 MARKERS USEFUL IN DIAGNOSIS OF HEMATOLYMPHOID NEOPLASMS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 23-3 KARYOTYPIC AND GENETIC CHANGES ASSOCIATED WITH NON-HODGKIN LYMPHOMA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 23-4 REACTIVE LYMPHADENOPATHY IN CHILDREN | |
|---|---|
|
of the paucity of lymphocytes. The morphologic features associated with persistent generalized lymphadenopathy (i.e., large and irregularly shaped follicles, follicular lysis, follicular involution) are distinctive but not specific for HIV infection, as they have been seen in 5% to 10% of otherwise entirely unremarkable lymph nodes obtained as part of carcinoma staging before the beginning of the AIDS era (20).
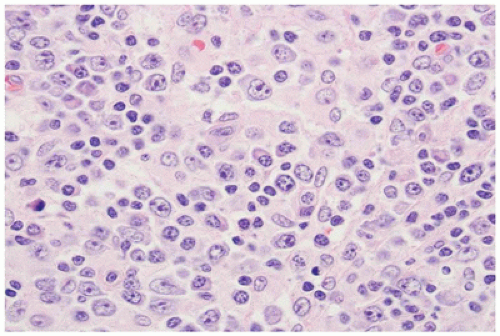
Normal landmarks—germinal centers and subcapsular and paratrabecular sinuses—are generally present but may be compressed or distorted by the immunoblastic proliferation. In very early cases of EBV infection, monocytoid B-cell proliferation may be prominent (36). Staining with CD20 and CD3 highlights the presence of a mixture of interfollicular B and T immunoblasts, respectively, and a polytypic pattern of light-chain expression is always seen. The Reed-Sternberg-like cells are characteristically CD20+ and CD15- and show variable reactivity for the activation antigen CD30 as well as markers of EBV infection such as latent membrane protein (LMP1) or EBV-encoded RNA transcripts (EBER) (37). Difficult cases may exhibit sheet-like arrays of immunoblasts, a brisk mitotic rate, or extensive necrosis and may closely mimic large-cell lymphoma. In such cases, examination of the peripheral blood smear for atypical lymphocytes, viral serology, and immunohistochemistry to better define architectural preservation and establish the presence of EBV is helpful.
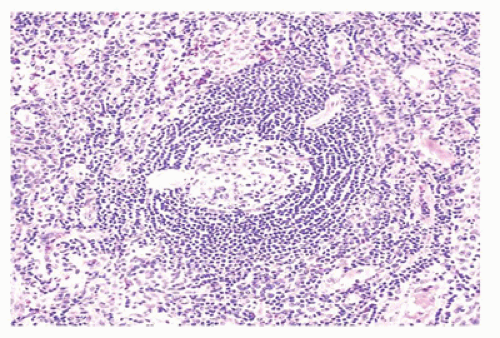
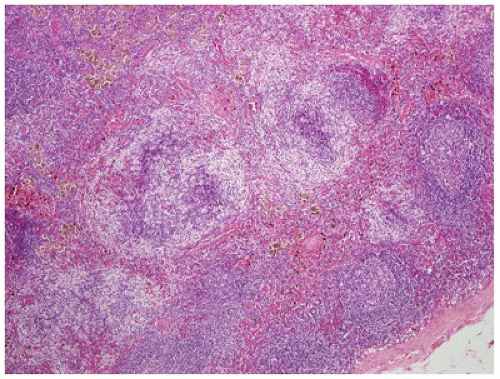
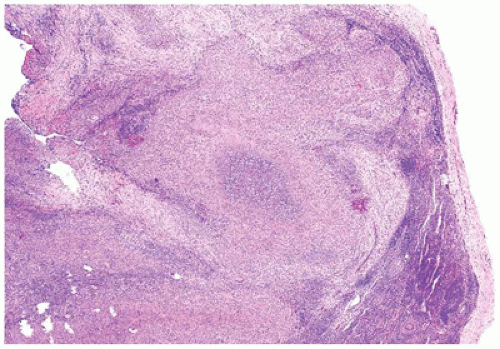 FIGURE 23-12 • The microabscesses in cat-scratch disease have a serpiginous or stellate contour. (Hematoxylin and eosin stain 4× magnification.) |
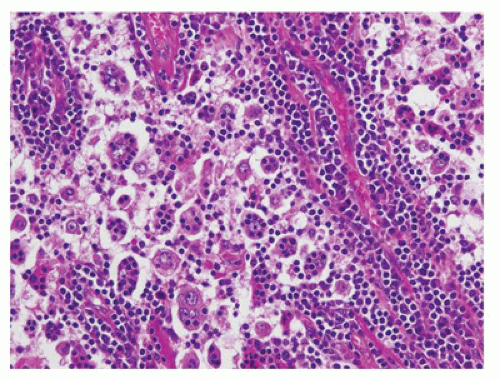
the MPT64 mycobacterial antigen (more specific for the Mycobacterium tuberculosis complex) (66,67). The remainder of cases may be diagnosed via one of several polymerase chain reaction-based techniques (68,69,70,71,72) or microbiologic culture. Most of these techniques, except for culture, have the advantage of being applicable to fresh as well as paraffin-embedded tissue and may be performed using cytology specimens, core needle biopsies, or whole lymph node biopsies. Other causes of caseating and noncaseating granulomatous lymphadenitis include nonmycobacterial infections and neoplastic disease, including peripheral T-cell lymphoma, NLPHL, and classical Hodgkin lymphoma (CHL).
ALPS, which should be taken into account in the evaluation of unusual cases (53,87,88,89). A subset of SHML shows features of IgG4-related disease, and an overlap between certain aspects of the two diseases has been suggested (90).
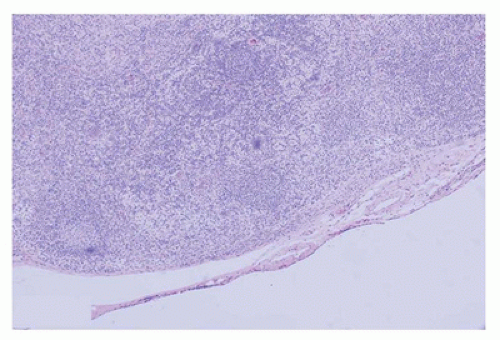
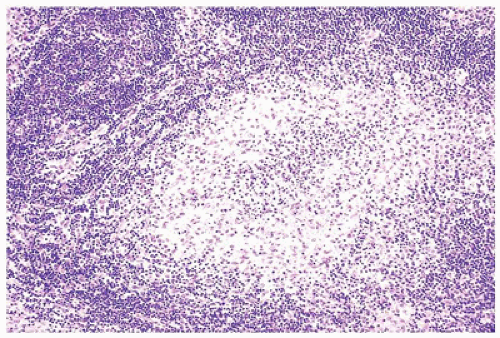
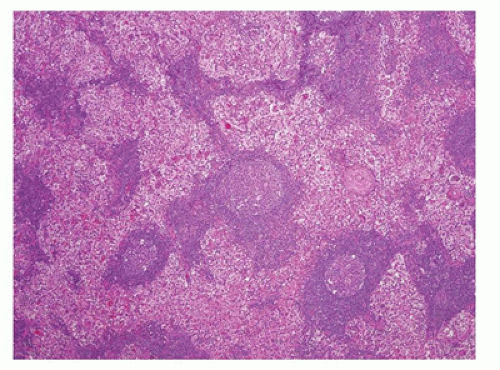 FIGURE 23-14 • As a result of massive sinusoidal expansion by histiocytes, germinal centers are compressed in Rosai-Dorfman disease (SHML). (Hematoxylin and eosin stain 4× magnification.) |
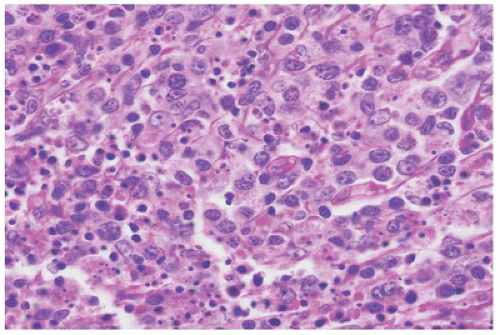
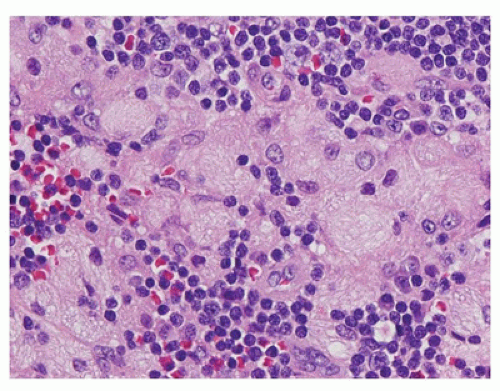
may have a pink mottled appearance because of infiltrating histiocytes and Langerhans cells (S100+, CD1a+, CD207+), some of which may contain coarsely granular brown-black melanin pigment (melanophages) that is positive on Fontana-Masson staining. Occasional hemosiderin pigment is also seen. FNA of lymph nodes with dermatopathia shows large clusters of histiocytes, histiocytes with melanin pigment, few tingible body macrophages, and histiocytes with elongated or grooved nuclei (94).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree