URINARY TRACT CYTOLOGY: ITS ACCOMPLISHMENTS AND FAILURES IN HISTORICAL PERSPECTIVE
Examination of urine belongs among the oldest medical procedures in the history of mankind. Ancient Egyptians were aware of the importance of bloody urine in the diagnosis of bladder disorders that were later identified as cancer caused by the parasite
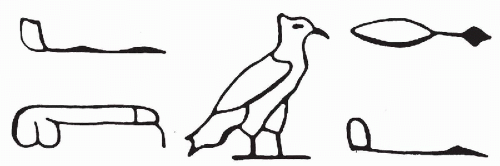
Schistosoma haematobium. As noted in an important historical contribution by Badr (1981), the papyrus of Kahun, dated 1900 years B.C., contains a hieroglyphic describing hematuria (
Fig. 22-1). The gross inspection of urine (often collected in special containers) or “uroscopy” was an important diagnostic procedure for many centuries.
Fisman (1993) presented an amusing account of the role played by uroscopy in 17th century England and pointed out that this practice persisted until the early years of the 20th century. In 1856, Wilhelm Duschan Lambl, a
Czech physician, published a remarkable paper on the use of the microscope for the examination of the urinary sediment at the bedside. Lambl described and illustrated a number of bladder conditions, including cancer (
Fig. 22-2). This was the beginning of contemporary cytology of the urinary sediment. The reader is referred to other sources for a detailed description of these early events (
Koss, 1995).
Nearly 150 years after the publication of Lambl’s contribution on the cytology of the urinary sediment, the benefits and limitations of this method of diagnosis are still poorly understood by urologists and pathologists. It would be a safe bet that the opinions of individual urologists may vary from total indifference to the method as worthless in clinical practice, to the rare enthusiastic endorsement, with the majority expressing a moderate degree of interest in a method of occasional value.
The problem with cytology of the urinary tract is the lack of basic understanding of the accomplishments and limitations of the method and of the pathologic processes accounting for it. As will be set forth in this and the next chapter, it is unrealistic to expect that the cytologic method will serve to recognize the presence or recurrence of low-grade papillary tumors. It is equally unrealistic to expect that cytology of urine, or of the various ancillary sampling procedures, will help in differentiating low-grade papillary tumors from other space-occupying lesions of the renal pelvis or ureter. This accounts for the introduction of numerous noncytologic methods of diagnosis by commercial companies, discussed in the next chapter.
On the other hand, cytologic techniques are highly effective in detection and diagnosis of high-grade malignant tumors, particularly flat carcinomas in situ, which are the principal precursor lesions of invasive urothelial cancer. Cytology of urine is also valuable in the recognition of various viral infections, particularly human polyomavirus, and the effects of various therapeutic procedures. In our judgment, cytology of the urinary tract is one of the most important diagnostic methods in urologic oncology, provided:
It is used properly by the urologist under well-defined circumstances and for well-defined reasons.
It is performed by a laboratory competent in processing and interpretation of such specimens.
The urologist and the pathologist understand the limitations of the method and are familiar with sources of error.
Application of Cytology in Forensic Sciences
An unusual application of cytology is the
identification of female cells in postcoital swab smears of the penis. Fluorescence in situ hybridization techniques were used to record female cells with two X chromosome signals (
Collins et al, 2000). The technique may be helpful in proving sexual contact in cases of presumed rape.
ANATOMY
The urinary tract is composed of the
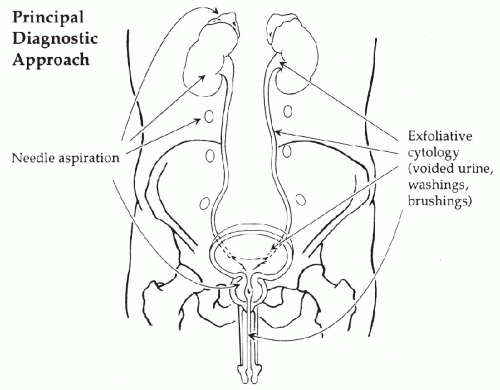
kidneys, ureters, urinary bladder, and urethra. The position of these organs in the abdominal cavity and the methods of cytologic sampling are shown in
Figure 22-3.
The
two kidneys are fist-sized, encapsulated organs located laterally in the retroperitoneal space. The principal function of the kidney is to
filter blood and eliminate harmful products of metabolism and other impurities that are excreted in
urine. The bulk of the kidney is constituted by the filtering apparatus or
nephrons, each composed of the principal filtering device, or the
glomerulus, connected to a series of
tubules. The filtrate generated by the glomeruli
undergoes many modifications in the tubulus until the final product of the filtration process, or the
urine, is excreted into the
renal pelvis, whence it travels through the
ureters to the
bladder.The kidney is essential to the maintenance of osmotic equilibrium in the blood. It also contributes to the regulation of blood pressure and has several other ancillary functions. It is beyond the scope of this book to provide details on the complex structure and function of the kidneys and interested readers are referred to specialized sources for further information.
The ureters are firm cylindrical structures, about 20 to 25 cm in length and about 0.5 cm in diameter. In their course toward the bladder, the ureters cross the pelvic brim and enter the bony pelvis, and thence the urinary bladder. In the female, the ureters pass near the lowest segment of the uterus to reach the bladder. This relationship is important in patients with invasive cancer of the uterine cervix that can surround and obstruct the ureters.
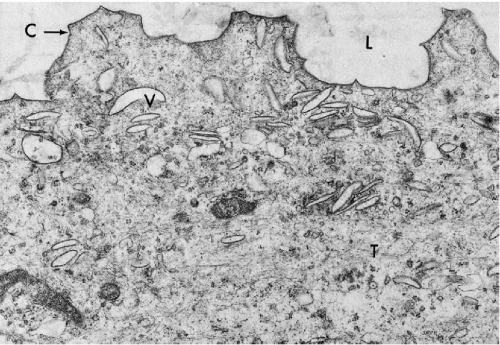
The bladder is a balloon-shaped organ composed from inside out of an epithelium, a connective tissue layer known as lamina propria, and an elastic muscular wall (muscularis propria). These component tissues work in unison to allow expansion of bladder volume while accumulating urine and collapse with voiding. Under pathologic circumstances, the bladder is capable of accommodating up to several liters of urine without rupture.
Lamina propria is a thin layer of connective tissue supporting the urothelium. It is rich in vessels and in most individuals, but not all, contains an interrupted thin layer of smooth muscle cells (muscularis mucosae). The nests of Brunn and the cysts of cystitis cystica are located within the lamina propria. Muscularis propria, or the principal muscle of the bladder, is composed of two thick concentric layers of smooth muscle, in continuity with the muscular wall of the ureters.
The embryologic derivation of the bladder is in part from the cloaca, or the terminal portion of the embryonal intestinal tract. A vestigial organ, the urachus, a remnant of the embryonal omphaloenteric duct, connects the bladder dome with the umbilicus. Other parts of the bladder are derived from the genital tubercle. This dual embryonal origin accounts for the variety of epithelial types that may occur in the bladder (see below). The basal portion of the urinary bladder contains the trigone, a triangular area with the apex directed forward to the urethra. The two ureters enter the bladder at the posterior angles of the trigone. The urine passes from the bladder into the urethra, which begins at the apex of the trigone. The important anatomic relationships of the trigone differ between females and males. In the female, the trigone overlies the vesicovaginal septum and the vagina, whereas in the male, the immediately underlying organs are the prostate and seminal vesicles. It is evident that cancers of these various organs may extend to the trigone and vice-versa.
The
female urethra has only a very short course and opens into the upper portion of the
vaginal vestibule, somewhat behind and below the clitoris (see
Chap. 8). In the
male, the urethra runs
across and through the prostate and enters the
penis. The anatomy of the prostate is discussed in
Chapter 33.
CYTOLOGY OF THE LOWER URINARY TRACT
As indicated in the anatomic diagram (see
Fig. 22-3), there are two principal methods of cytologic sampling of the urinary tract:
exfoliative cytology based on voided urine, washings or brushings of the epithelial surfaces, and
needle aspiration techniques of solid organs, the kidneys, adrenals, retroperitoneum, and prostate. In this chapter, only the exfoliative cytology of the epithelial surfaces of the lower urinary tract is described. Aspiration biopsy of the solid organs is described in
Chapters 33 and
40.
Methods of Specimen Collection
The principal methods of specimen collections are:
The selection of the method of specimen collection and processing depends on clinical circumstances and the goal of the examination. The advantages and disadvantages of the various methods are summarized in
Table 22-2.
Voided Urine
This is by far the easiest and least expensive method of cytologic investigation of the urinary tract. The technique is valuable as a preliminary assessment of a broad spectrum of abnormalities of the urethra, bladder, ureters and renal pelves and, under special circumstances, of the kidney and prostate.
Urine is an acellular liquid product of renal excretory function. As the liquid passes through the renal tubules, renal pelvis, ureter, bladder, and urethra, it picks up desquamating cells derived from the epithelia of these organs. Inflammatory cells, erythrocytes, and macrophages are frequently seen. Voided urine normally has an acid pH and a high content of urea and other organic components; therefore, it is not isotonic. Consequently, the urine is not a hospitable medium for desquamated cells, which are often poorly preserved and sometimes difficult to assess on microscopic examination.
Collection
Morning urine specimens have the advantage of highest cellularity, but also the disadvantage of marked cell degeneration.
A specimen from the morning’s second voiding is usually best. Three samples obtained on 3 consecutive days are diagnostically optimal (
Koss et al, 1985). Naib (1976) recommended hydration of patients to increase the yield of desquamated cells (1 glass of water every 30 minutes during a 3-hour period).
Processing
Unless the urine is processed without delay, the addition of a
fixative is recommended. In our hands, the
best fixative is 2% polyethylene glycol (Carbowax) solution in 50% to 70% ethanol (
Bales, 1981). To achieve best results, the patient should be provided with a 250 to 300 ml wide-mouth glass or plastic container one-third filled with fixative. This makes it convenient for
home collection of samples.
The urinary sediment can be processed in a variety of ways. The specimen can be centrifuged for 10 minutes at moderate speed and a
direct smear of the sediment made on adhesive-coated slides. The urine
can be filtered using one of the commercially available filtering devices, either for
direct viewing of cells on the surface of the filter, or after transferring the filtered cells to a glass slide by imprinting them
(reverse filtration). Alternatively, the cellular sediment can be placed on an adhesive-coated slide by use of a
cytocentrifuge, preferably using the
method developed by Bales (1981) in our laboratory. Several commercial methods of preparation of the urinary sediment have been developed within recent years. Urine sediment preparation by ThinPrep has been reported by
Luthra et al (1999) as giving satisfactory results. Both ThinPrep and SurePath gave satisfactory results to Wright and Halford (2001). Still, these methods
may modify the appearance of urothelial cells, particularly their nuclei. For further details on sample processing, see
Chapter 44. The use of
phase microscopy (
de Voogt et al, 1975) and of
supravital stains (
Sternheimer, 1975) in the assessment of urine cytology has been suggested. Neither method received wide acceptance.
Catheterized Urine
The specimens are collected via a catheter and processed as described above for voided urine.
Direct Sampling Techniques
Bladder Washings or Barbotage
This procedure may be used to obtain
cellular specimens of well-preserved epithelium from patients at high risk for development of new or recurrent bladder tumors. It is the specimen of choice for
DNA ploidy analysis of the urinary epithelium, discussed in
Chapters 23 and
47. The bladder should first be emptied by catheter. Bladder barbotage is then best performed
during or prior to cystoscopy by instilling and recovering 3 to 4 times 50 to 100 ml of normal saline or Ringer’s solution. The procedure can also be performed through
a catheter but it is uncomfortable, particularly for male patients, and the results are less satisfactory.
Retrograde Catheterization of Ureters or Renal Pelves
This procedure is used
to establish the nature of a space-occupying lesion of ureter or renal pelvis, observed by radiologic techniques. The most common application of the procedure is in the
differential diagnosis between a stone, a blood clot, or a tumor. Other rare, space-occupying lesions of the
renal pelves are
inflammatory masses, angiomas, and congenital aberrations of the vascular bed. In the
ureter, there may be lesions caused by a
tumor, a stricture, or extraneous pressure.Another important application of this technique is the localization of an occult malignant tumor diagnosed in voided urine sediment but not found in the bladder. The purpose is to determine whether the tumor can be localized in the left or right kidney or ureter. For urine collection, separate catheters must be used for each side to avoid cross-contamination. The best results are obtained by inserting the catheter to a depth of 3 to 4 cm into the ureters and by placing the other tip of the catheter in a container with fixative. From 10 to 30 minutes may be needed to collect 5 to 10 ml of urine necessary for diagnosis. Although the procedure may be tedious to the patient, it is quite efficient in localizing the lesion.
The Direct Brushing Procedure
This procedure is used in the investigation of space-occupying lesions in the ureters or renal pelves. Brushing is performed through a ureteral catheter. The indications are the same as listed for retrograde catheterization.
Processing of Direct Samples
Bladder washings or barbotage specimens may be processed in a manner similar to voided urine, discussed above.
Retrograde catheterization specimens, if liquid, are processed in a similar manner.
Direct brush specimens are usually prepared in the cystoscopy suite by the urologist and submitted as
smears. For
optimal preservation, the smears should be immediately fixed in 50% ethanol for at least 10 minutes. Alternatively, the
brushes can be placed in a 50% alcohol fixative and forwarded to the laboratory for further processing. See
Chapter 44 for further comments on processing of this material.
Cellular Components of the Urinary Sediment
An understanding of the complexities of the normal cell population of the urinary sediment under various clinical circumstances is an important first step for proper diagnostic utilization of cytology of the urinary tract. Methods of sample collection and processing have a major impact on the interpretation of the cytologic images. As always, information on clinical circumstances and clinical procedures leading to the collection of the cytologic samples may prevent major errors of interpretation, particularly in low-grade urothelial tumors.
The urinary sediment contains:
Cells derived from the urothelium and its variants
Cells derived from renal tubules
Cells derived from adjacent organs
Cells extraneous to the urinary tract, such as macrophages and blood cells
Normal Urothelial Cells
Normal urothelial cells have several features that set them apart from other epithelial cells. The
cells vary greatly in size: the superficial umbrella cells are often very large and may contain multiple nuclei, whereas epithelial cells from the deeper layers of the urothelium are much smaller and usually have a single nucleus. Another important general feature of urothelial cells is their tendency to desquamate in
large, complex clusters, particularly after instrumentation. It must be stressed, once again, that
the appearance of the sediment varies according to the method of collection.
Superficial Umbrella Cells
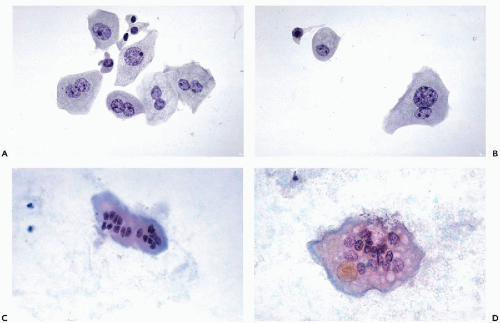
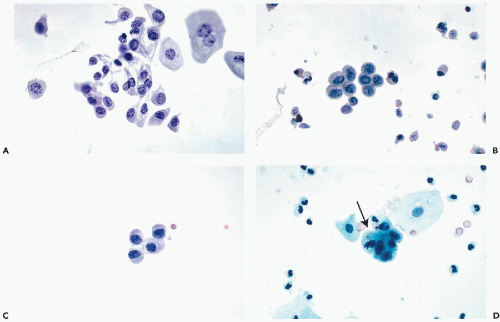
The
size of the umbrella cells is variable and depends to some extent on the number of nuclei. The average
mononucleated umbrella cells measure from
20 to 30 μm in diameter and, hence, are much larger than the cells from deeper epithelial layers (see below).
Multinucleated umbrella cells may be
substantially larger (
Fig. 22-7). These cells are usually
flat and polygonal and usually have one
sharply demarcated, and sometimes angulated, surface. The
abundant cytoplasm is thin and transparent, sometimes faintly vacuolated, and may contain
fat (
Masin and Masin, 1976) or
mucus-containing vacuoles (
Dorfman and Monis, 1964). Sometimes, the nature of the vacuoles cannot be ascertained (
Fig. 22-8A).
The nuclei also vary in size. In
mononucleated cells, the
spherical or oval nuclei may measure from
8 to 20 μm in diameter, depending on the size of the cell. The large nuclei reflect a tendency of urothelial cells to form
tetraploid or even
octaploid nuclei (Levi et al, 1969; Farsund, 1974). This tendency to polyploidy appears to be part and parcel of the pattern of normal urothelial differentiation but its mechanism is unknown. These features of normal umbrella cells are important in DNA measurements (
Wojcik et al, 2000). In well-preserved umbrella cells, the nuclei are
faintly granular but may contain prominent basophilic chromocenters that should not be
confused with large nucleoli. Using some of the
newer methods of semiautomated processing the chromocenters may be eosinophilic, accounting for additional difficulties in the interpretation of the samples (
Fig. 22-8B).
In some samples processed by commercial methods, we have observed peculiar condensation of nuclear chromatin, mimicking mitotic figures (
Fig. 22-8C).
Multinuclated umbrella cells also vary in size. Most cells are binucleated and contain either nuclei of normal size and configuration or smaller. Other cells have
multiple nuclei of variable sizes (see
Fig. 22-7C).
Large and small nuclei often occur side by side within the same cell. Still other umbrella cells may contain
10 or more small nuclei and appear as multinucleated giant cells (see
Fig. 22-8C,D). Umbrella cells derived directly from
the ureters are often much larger with many more nuclei than in cells derived from the bladder. Occasionally, the nuclei in the large superficial cells are
clumped and degenerated and may be mistaken for nuclei of cancer cells, an important source of diagnostic error (see
Fig. 22-8D).
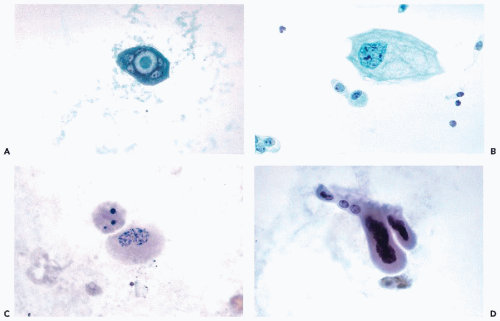
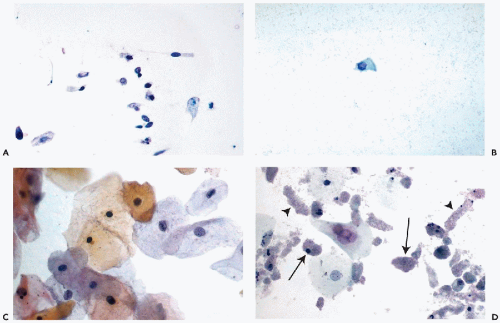
Cells from the Deeper Layers of the Urothelium
Urothelial cells originating in the deeper layers of the urothelium, are rarely seen in voided urine, but are common in specimens obtained by or collected after instrumentation. These cells are much smaller than umbrella cells and are
comparable in size to small parabasal cervical squamous cells and unlike the superficial cells,
show little variation in size. When fresh and well preserved, these cells have
sharply demarcated transparent cytoplasm that is often
elongated and whip-shaped when the cells are removed by instruments. The cytoplasm is stretched at points of desmosomal attachments to neighboring cells, a phenomenon also observed in metaplastic cervical cells (see
Chap. 8). The
finely granular nuclei may contain single chromocenters, mimicking nucleoli (
Fig. 22-9A,B).
Occasionally, the nuclei may be pyknotic, particularly in brushings (
Fig. 22-9C).
Similar cells in voided urine may have pale, transparent nuclei. Occasionally, mitotic figures may be noted, particularly in
urinary sediment obtained after a diagnostic or therapeutic surgical procedure (
Fig. 22-9D).
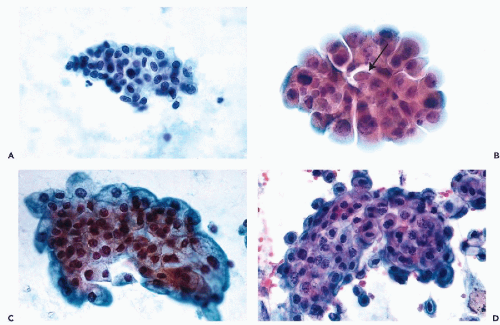
Clusters of Urothelial Cells
A very important feature of normal urothelium is its propensity to desquamate in fragments or clusters that are sometimes very complex. Although this feature is markedly enhanced in urine samples obtained by bladder catheterization, lavage, or any type of instrumentation, urothelial cell clusters may also occur in spontaneously voided urine. It appears that even abdominal palpation, the slightest trauma, or inflammatory injury to the bladder may enhance the shedding of clusters. The clusters may be small and flat, composed of only a relatively few clearly benign cells (
Fig. 22-10A), or much larger and composed of
several hundred superimposed cells. The clusters may round up and
appear to be spherical, oval, or “papillary” in configuration (
Fig. 22-10B). Occasionally, they are more
complex (
Fig. 22-10C) and sometimes
distorted during smear preparation. The distortion
may be increased if frosted slides are used. The periphery of such clusters should be carefully examined under high magnification of the microscope.
On close inspection, the edge of the clusters is sharply demarcated, and the normal component cells of the urothelium may be readily observed (
Fig. 22-10D). It must be noted that in urine sediment prepared by the
ThinPrep method, nuclear chromocenters sometimes stain pink or red and thus may be considered to be “atypical” or even malignant.
It is paramount in urinary cytology to avoid making the diagnosis of a papillary tumor based on the presence of clusters. For further discussion of cytology of bladder tumors, see
Chapter 23.
Cytologic Expressions of Epithelial Variants
It has been pointed out above that several epithelial variants may occur in bladder epithelium.
Intestinal-type epithelium may be the source of columnar, sometimes mucus-producing cells that are found in bladder washings and catheterized specimens but are uncommon in voided urine (
Fig. 22-11A). These cells have a generally clear cytoplasm and spherical, finely granular small nuclei.
Harris et al (1971) first described
ciliated columnar cells in bladder washings (
Fig. 22-11B).
There are no specific cytologic findings corresponding to Brunn’s nests and cystitis cystica. Dabbs (1992) published his observations in renal pelvic washings in a case of
pyelitis cystica but the findings, as illustrated, showed only clusters of normal urothelial cells.
Squamous epithelium sheds squamous cells of various degrees of maturity (
Fig. 22-11C). The finding
is exceedingly common and normal in adult women but is somewhat less frequent in men. In women, the urinary sediment may be used to assess their hormonal status
(“urocytogram”), as discussed in
Chapter 9. In both sexes, the harmless
squamous epithelium may become fully keratinized (leukoplakia), presumably as a consequence of chronic irritation. The cytologic findings and significance of leukoplakia of the bladder are discussed below.
Renal Tubular Cells
Renal tubules are
lined by a single layer of epithelial cells that vary somewhat in configuration according to the segment of the tubule. Of special interest in urine cytology is the terminal part of the tubular apparatus or the
collecting ducts, lined by a single layer of cuboidal to columnar epithelial cells with clear cytoplasm and small spherical nuclei. These cells may be observed in the urinary sediment under various circumstances.
Renal tubular cells may be numerous whenever there is some
insult to the renal parenchyma, for example, after an
intravenous pyelogram. They may be found after an episode of
hematuria or hemoglobinuria. The small cells are
poorly preserved, cuboidal or columnar in shape, and are characterized by
small pyknotic nuclei and granular cytoplasm (
Fig. 22-11D). The presence of numerous, well-preserved tubular cells is of importance in monitoring renal transplant patients (see below). The renal tubular cells have
phagocytic properties and may store the
dyes used in intravenous pyelography, which are visualized as a yellow pigment in the cytoplasm.
Khalil et al (1999) described
large, vacuolated renal tubular cells with some similarity to macrophages, in the voided urine sediment of a patient with a rare disorder,
osmotic nephrosis. The patient was treated with intravenous immunoglobulins stored in the cytoplasm. The identity of the cells was confirmed by immunochemistry and electron microscopy.
The presence of renal tubular cells in the urinary sediment must be correlated with clinical circumstances and does not necessarily indicate the presence of a renal disorder.
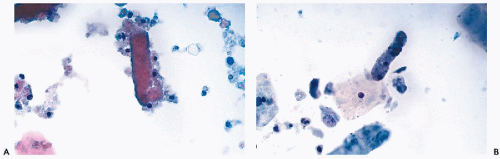
Renal Casts
Accumulation of various proteins, erythrocytes, leukocytes, necrotic cells, and cellular debris molded into longitudinal cylindrical structures are known as renal casts. It is often assumed that the presence of casts indicates a severe renal disorder. However, carefully collected and fixed urinary sediment samples often reveal renal casts in the absence of disease.
In voided urine specimens collected in a 2% polyethylene glycol (Carbowax) solution in 70% ethanol (
Bales, 1981; see
Chap. 44),
renal tubular cells and casts are well preserved and observed with unexpected frequency, even in patients without overt evidence of renal pathology.The renal tubular casts are either
hyaline or granular. The
hyaline casts are composed of
homogeneous eosinophilic protein material, sometimes with a few renal tubular cells attached at the periphery (
Fig. 22-12A). The
granular casts are composed of
cell debris mixed with degenerating renal tubular cells with granular cytoplasm (
Fig. 22-12B; see also
Fig. 22-11D). Such casts are very common in renal transplant patients during episodes of rejection (see below) but may also be observed after urography during the period of elimination of the dye (
Fischer et al, 1982). The presence of casts must be correlated with clinical data as it may indicate the presence of a renal parenchymal disorder.
Cells Originating in Adjacent Organs
Cells from the Prostate, Seminal Vesicles, and Testis
These cells may be observed in the sediment of voided urine
after a vigorous prostatic palpation or massage. The dominant component of such specimens are usually
spermatozoa and their precursor cells, including
spermatogonia, characterized by larger dark nuclei. The
normal prostatic glandular cells are difficult to recognize as they are small and have few distinguishing characteristics. By far, the most important cells in such sediments are
cells derived from seminal vesicles, which are
large and may have large, irregular, hyperchromatic but homogeneous nuclei, mimicking cancer cells. They are almost invariably degenerated when expelled and are recognized by the presence of
yellow cytoplasmic lipochrome granular pigment. For further description of these cells in health and disease, see
Chapter 33.
Cells From the Female Genital Tract
The urinary stream may pick up cells from the vagina and the vulva. The most common are
normal squamous cells (see above),
but abnormal cells reflecting neoplastic processes in the female genital tract may also occur (see
Chap. 23).
Other Cells
Blood Cells
Besides the urothelial and squamous cells, the normal urinary sediment may contain a few leukocytes. It is generally assumed that normal urinary sediment does not contain any red blood cells. Yet Freni (1977), using a careful collection technique, documented that a few erythrocytes may be observed in virtually all healthy adults. In 8.8% of this healthy population, there were 10 erythrocytes per single, high-power field. These observations were important because the presence of microscopic blood in urine has been suggested as a means of detecting bladder tumors.
Microhematuria
Microhematuria is by definition, the presence of erythrocytes in urine. Because normal people may show up to 10 erythrocytes per high-power field, the diagnosis should not be rendered unless the number of erythrocytes is higher.
Cohen and Brown (2003) proposed a much lower threshold for the diagnosis of microhematuria, namely two erythrocytes per high-power field or a positive dipstick evaluation for hemoglobin. Microhematuria in asymptomatic persons has been the subject of several studies. Unfortunately, the populations studied were different and therefore no simple conclusion can be drawn. In an earlier study by
Greene et al (1956), 500 Mayo Clinic patients with microhematuria were investigated and 11 of them were found to have cancer (7 of bladder and 2 of kidney). Most other patients had trivial and incidental disorders. In a study by
Carson et al (1979) of 200 Mayo Clinic patients referred for a urologic workup, 22 (11%) had a tumor of the bladder and 2 had carcinoma of the prostate. It is of note in the Carson study, that
synchronous cytologic examination of urine was positive in 9 patients with occult carcinoma in situ and negative in 5 patients with low-grade papillary tumors (see
Chap. 23). On the other hand, in a study of
1,000 asymptomatic male Air Force personnel,
Froom et al (1984) found
microhematuria in 38.7%. In only one subject, a “transitional cell carcinoma,” not further specified, was observed. In a randomized 1986 study, Mohr et al observed microhematuria in 13% of asymptomatic adult men and women, with neoplasms of the bladder in 0.1 % and of the kidney in 0.4% of the population studied.
Bard (1988) observed no significant disease in 177 women with microhematuria, followed for more than 10 years.
The initial views on the significance of microhematuria suggested an aggressive investigation of all patients with this disorder. More recent opinions, notably by
Mohr et al, Messing et al (1987),
Bard (1988), and
Grossfeld et al (2001) suggest that a
conservative follow-up of most asymptomatic patients is appropriate, with cystoscopic work-up reserved for the patients with persisting significant hematuria or other evidence or suspicion of an important urologic disorder. Carson’s study suggests that a
cytologic follow-up may be helpful in some patients. Similar guidelines were more recently proposed by the American Urological Association (
Grossfeld et al, 2001).
As
Messing et al (1987) noted, microhematuria is a sporadic
event that may occur intermittently and may not occur at all in patients with significant disease. Therefore, it seems quite unlikely that microhematuria may be used as a screening test for bladder tumors.
Renal versus Nonrenal Origin of Erythrocytes
The issue of differentiation of microhematuria caused by parenchymal renal disease, such as glomerulonephritis, versus microhematuria of other origin is controversial.
Rathert and Roth (1991) suggested that a microscopic examination of either very fresh or rapidly fixed voided urine sediment does allow the separation of the two cell types. In phase microscopy, erythrocytes of renal origin were characterized by a
dense periphery in the form of a double ring, and an “empty” center. Another manifestation of renal hematuria may be
partial breakdown of erythrocytes with the appearance of small, irregular, oddly shaped cells. Other extrarenal erythrocytes acquire features akin to poikilocytosis, with the
periphery of the erythrocytes covered with spike-like excrescences or spherical protrusions.Mohammed et al (1993) and
Van der Snoek et al (1994) (among others) suggested that the presence of
“dysmorphic” erythrocytes, that is erythrocytes with abnormalities of shapes, was suggestive of a renal parenchymal disorder, whereas
“isomorphic erythrocytes (i.e., red blood cells of normal shape) were representative of nonrenal origin of hematuria. Mohammed, using phase microscopy, indicated that the cut-off point of 20% of dysmorphic erythrocytes had a sensitivity of 90% and specificity of 100%. Van der Snoek used a cut-off point of 40%, achieving a sensitivity of 66.7%. The specificity of these observations was debated, among others, by
Pollock et al (1989),
Favaro et al (1997),
Zaman and Proesmans (2000). Most recently,
Nguyen (2003) examined the urinary sediments in 174 patients with various forms of glomerular disease and observed doughnut-shaped, target- or bleb-forming erythrocytes (collectively named
G1 or GIDE cells) in a substantial proportion of cases. Nguyen proposed that the presence of GIDE cells above 10% of the erythrocyte population was a “specific diagnostic marker for glomerular disease.” Unfortunately, the study did not include control patients with other possible causes of microhematuria and, thus, its specificity must still be proved.
Eosinophiluria
The presence of
bilobate eosinophils in urine may be an indication of a drug-induced or spontaneous eosinophilic cystitis (see below).
Nolan et al (1986) suggested the use of
Hansel’s stain (methylene blue and eosin-Y in methanol) to facilitate the recognition of eosinophils.
Acellular Components
Crystals
Urate crystals are commonly seen in poorly fixed urine specimens. The precipitation of urates occurs with a change in the pH of the urine, usually occurring after collection. The
crystals are usually semi-transparent, of odd shapes, and have no diagnostic significance, except that they may completely obscure cells present in the specimen.
Triple phosphate crystals are transparent, often rectangular.
The star-like uric acid crystals derived from stones, are rarely seen. From time to time, other crystals may be observed, such as the needle-like crystals of tyrosine or
hexagonal crystals of cystine. Certain drugs, notably sulfonamides, may also form crystals of specific configuration. The reader is referred to
Naib’s book (1985) for a detailed description of uncommon crystals observed in the urinary sediment.
Renal casts were discussed above.
Contaminants
Urinary samples may sometimes contain
surgical powder in the form of crystalline precipitates. Cotton threads may also occur. Occasionally, the
brown, septated fungus of the species Alternaria, a contaminant from the water supply, may be observed. For a description and illustrations of this fungus, see
Chapter 19.