The Lower Respiratory Tract in the Absence of Cancer: Conventional and Aspiration Cytology
Myron R. Melamed
ANATOMY
The respiratory tract serves the dual purpose of supplying oxygen to and removing carbon dioxide from the circulating blood. This exchange takes place at the level of the pulmonary alveoli. Oxygen-rich air is inhaled, and carbon-dioxide rich air is exhaled through a complex series of conduits extending from the upper or cranial portions of the respiratory tract (e.g, the nasal cavity and the mouth*) to the thinwalled terminal alveoli of the lung via the larynx, trachea, and bronchi. The trachea and main bronchi are rigid, resisting collapse as pressures within the thorax change during respiratory movements. The musculature of the thorax and the diaphragm initiate inspiration by expanding the thoracic cage, thereby creating negative pressure within the pleural cavity that is transmitted to the elastic lungs. A very thin layer of fluid facilitates the movement of the pleural surfaces against each other (see anatomy of the serous cavities in Chap. 25).
The respiratory tract may be roughly divided into three portions. The cranial portion is supported by the bones of the skull and the cervical vertebrae; it comprises the nasal cavity and the paranasal sinuses, the buccal cavity, and the pharynx. The intermediate portion is composed of the larynx, trachea and the main bronchi; it stretches from the larynx to the hilus of each lung. The third portion is the lung proper, composed of lobar, segmental and smaller bronchi, and the alveolar system with its extraordinarily rich blood supply (Fig. 19-1A). A brief discussion of the various anatomic components follows.
Upper Airway
The nasal cavity functions principally as a conduit for inspired air, but also serves in warming and moistening the air, and trapping larger dust particles. It is subdivided by the turbinate bones into three compartments, of which the uppermost is partially lined by the olfactory mucosa containing receptors for the sense of smell. The middle and lower compartments are purely respiratory. All three nasal compartments communicate through small orifices directly into the paranasal sinuses. The nasal cavity opens posteriorly into the pharynx, a space demarcated posteriorly by the spine and its muscles, reaching upward to the base of the skull and downward to be in direct continuity with the esophagus and the larynx. Of importance within the pharynx is the presence of rich deposits of lymphoid tissue, especially the tonsils, located anterolaterally on each side of the pharynx, and the pharyngeal or third tonsil (adenoids) located posteriorly near the base of the skull. The mouth or buccal cavity also opens posteriorly into the pharynx; the tongue with its complex and exquisitely developed musculature occupies the central portion of the buccal cavity. The ducts of numerous salivary glands open into the buccal cavity, providing a constant flow of saliva.
Intermediate Airway
Inferiorly, the pharynx communicates with the larynx anteriorly and continues posteriorly as the esophagus. The epiglottis forms a lid capable of closing the larynx during the act of swallowing and thereby prevents entrance of food particles into the lower respiratory tract. The larynx is contained within a system of cartilages and is in direct continuity with the trachea, a semi-rigid tube kept open by C-shaped rings of cartilage that are incomplete posteriorly where the trachea is in contact with the esophagus. Within the thorax, approximately at the level of the fourth thoracic vertebra, the trachea divides into two main branches—the left and right mainstem bronchi.
Lower Airway
Each mainstem bronchus enters the corresponding lung accompanied by branches of the pulmonary artery and veins
in an area designated as the hilus. The left lung is partially separated by fissures into two lobes; the right lung has three lobes. Thus, the mainstem bronchi divide into two lobar bronchi on the left and three on the right. Subsequently, each lobar bronchus divides into segmental bronchi (10 on the right and 9 on the left; Fig. 19-1B), which undergo 18 dichotomous divisions into subsegmental bronchi and bronchioles that, in turn, form the thinwalled respiratory bronchioles, each of which opens into several alveoli. Although the lumina of individual bronchi become smaller with each bronchial division, the total aircarrying volume increases progressively to reach its greatest capacity at the level of the alveoli, which form the bulk of the pulmonary parenchyma.
in an area designated as the hilus. The left lung is partially separated by fissures into two lobes; the right lung has three lobes. Thus, the mainstem bronchi divide into two lobar bronchi on the left and three on the right. Subsequently, each lobar bronchus divides into segmental bronchi (10 on the right and 9 on the left; Fig. 19-1B), which undergo 18 dichotomous divisions into subsegmental bronchi and bronchioles that, in turn, form the thinwalled respiratory bronchioles, each of which opens into several alveoli. Although the lumina of individual bronchi become smaller with each bronchial division, the total aircarrying volume increases progressively to reach its greatest capacity at the level of the alveoli, which form the bulk of the pulmonary parenchyma.
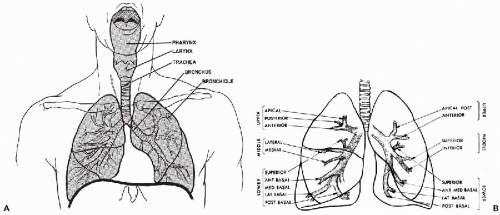 Figure 19-1 Diagrams of the respiratory tract. A. Upper respiratory tract. B. Lower respiratory tract, showing lobar and segmental branching of the bronchial tree. |
Each alveolus is a small, thin-walled sac, described in detail below. Capillary branches of the pulmonary artery run in the alveolar walls or alveolar septa, bringing blood that is poor in oxygen from the right ventricle and carrying away oxygenated blood in interlobular venules to pulmonary veins to the left atrium. The exchange of gases takes place across the alveolar wall. The lung itself is nourished by branches of the bronchial arteries that come from the aorta and follow the branching bronchi into the lung along with the pulmonary vessels, returning blood through the pulmonary veins.
Except at the hilus, the lungs are entirely surrounded by the visceral layer of the pleura.
HISTOLOGY OF THE NORMAL RESPIRATORY TRACT
Epithelial Lining
Two principal types of epithelium are encountered within the upper respiratory tract and the bronchial tree: nonkeratinizing, stratified squamous epithelium, which has no distinguishing features, and a characteristic respiratory epithelium. The olfactory mucosa, present in the uppermost portion of the nasal cavity, does not play a significant role in the cytology of the respiratory tract. The epithelia lining the respiratory alveoli and the alveolar macrophages will be described separately.
Squamous Epithelium
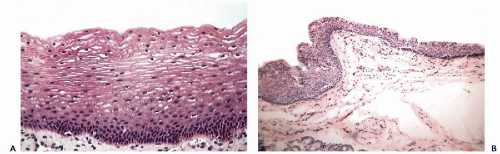
Stratified squamous epithelium lines the anterior portion of the nasal cavity, the mouth, tonsils, and central and lower portions of the pharynx. In general, the mucosa overlying and tightly adherent to bony structures, the hard palate, for example, and buccal mucosa that is subject to chronic irritation as in patients with poor dental hygiene, tends to form a superficial layer of keratin and therefore appears white; elsewhere throughout most of the mouth and oropharynx, it is nonkeratinizing (Fig, 19-2A). In the larynx, the upper or buccal aspect of the epiglottis is lined by nonkeratinizing stratified squamous epithelium, and the vocal cords are lined by a layer of thin, yet mechanically very resistant squamous epithelium (Fig. 19-2B). The remainder of the laryngeal mucosa may show islands of stratified squamous epithelium alternating with respiratory epithelium.
Respiratory Epithelium
Respiratory epithelium surfaces the major portion of the nasal cavity, the paranasal sinuses, the upper or nasal portion of the pharynx and adenoids, parts of the larynx, all of the trachea, and the bronchial tree (McDowell et al, 1978).
The respiratory epithelium is a pseudostratified columnar epithelium, characterized by the presence of ciliated columnar cells with interspersed mucus-secreting goblet cells. The term pseudostratified is used to describe epithelia
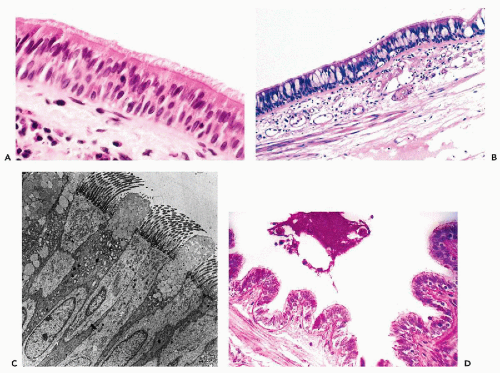
with nuclei located at different levels, hence the stratified appearance, although most cells are attached to the basement membrane. The cilia are anchored to the luminal surface of the bronchial cells by a row of points of attachment, combining to form a readily visible dark line or terminal plate (see Chap. 2). At their opposite end, where the columnar cells attach to the basement membrane, they are tapered, leaving a triangular space between the cells within which are small, triangular basal or reserve cells that are the source of epithelial regeneration. The basic structure of the respiratory epithelium is illustrated in Figure 19-3A.
with nuclei located at different levels, hence the stratified appearance, although most cells are attached to the basement membrane. The cilia are anchored to the luminal surface of the bronchial cells by a row of points of attachment, combining to form a readily visible dark line or terminal plate (see Chap. 2). At their opposite end, where the columnar cells attach to the basement membrane, they are tapered, leaving a triangular space between the cells within which are small, triangular basal or reserve cells that are the source of epithelial regeneration. The basic structure of the respiratory epithelium is illustrated in Figure 19-3A.
The goblet cells derive their name from an approximately triangular shape, resembling a wine goblet, with the nucleus placed at the narrow, basal end of the cell, while the clear supranuclear cytoplasm is distended by mucusforming small vacuoles (Fig. 19-3B). The number of goblet cells is variable. They may be numerous under certain pathologic circumstances, such as chronic bronchitis and asthma. The fine structure of ciliated columnar and goblet cells is shown in an electron micrograph in Figure 19-3C. The goblet cells produce a thin layer of mucus that carpets the surface of the ciliated epithelium. This mucus carpet (also known as the mucociliary escalator) captures respired dust particles and is kept moving by the coordinated motion of the beating cilia in the direction of the larynx where it is removed by coughing. This function is lost in patients who suffer from genetic abnormalities of ciliary structure and function known as immobile ciliary syndrome, discussed below.
Within the trachea and the main bronchi, the epithelium is truly stratified with two, three, or more layers of columnar cells, not all of which reach the surface. The cells that do not reach the surface have no cilia, an example of cellular differentiation determined by spatial arrangement. Goblet cells and ciliated cells progressively decrease in number in the smaller bronchial branches, and give way to nonciliated columnar and cuboidal cells. The epithelium of the smaller bronchioles is single layered and epithelial cells are low, columnar, or cuboidal.
The terminal bronchiolar epithelium includes Clara cells, nonmucus-secreting cells that produce surfactant (see below). They are characterized by protruding apical cytoplasm containing PAS-positive, diastase-resistant secretory material (Fig. 19-3D) and characteristic electron-dense, apical cytoplasmic granules (Cutz and Conen, 1971). They can be identified also by immunocytochemical staining with antibody to human surfactant-associated glycoproteins (Balis et al, 1985).
A small number of basally placed neuroepithelial cells known as Feyrter or Kulchitsky cells also are present, primarily at airway bifurcations. They are most numerous in fetal lungs but relatively sparse in the adult and are characterized by dense core neurosecretory granules in electron micrographs. In some individuals living at high altitudes or with chronic lung disease, there may be multiple minute hyperplastic nests of these neuroendocrine cells, which have been termed tumorlets. They have been shown to secrete a number of polypeptide hormones (McDowell et al, 1976b), including corticotropin that in one reported case was the cause of Cushing’s syndrome (Arioglu et al, 1998). The Kulchitsky cells are considered to be the parent cells of carcinoid tumors (see Chap. 20).
The terminal bronchioles open into a vestibule-like respiratory bronchiole with nearly flat epithelium from which respired air enters several communicating alveoli.
The Alveoli
The roughly spherical thin-walled alveolus is the functional unit of the lung, where exchange of oxygen and carbon dioxide takes place between air space and capillary. Ultrastructural studies have shown the wall of the alveolus to be surfaced by two types of epithelial cells, pneumocytes type I and pneumocytes type II, represented schematically in Figure 19-4A. Pneumocytes type I are flattened cells, few in number, with extremely attenuated cytoplasm that surfaces at least 90% of the alveolar wall. They have few cytoplasmic organelles, are metabolically inactive, cannot be visualized in conventional histologic sections, and are not capable of regeneration.
The remaining 10% of the alveolar surface is occupied by more plump, rounded, or cuboidal pneumocytes type II. Although they too are scarcely (if at all) visible in conventional histologic sections of normal lung, these cells are capable of proliferating and can become hyperplastic in a broad variety of chronic inflammatory lung diseases. They are the source of regenerating pneumocytes type I. They express epithelial cytokeratins (Fig. 19-4B), are metabolically very active and, like the Clara cells, they synthesize alveolar surfactant, a detergent-like protein that lines the inner surface of the alveoli, lowering surface tension and preventing collapse of the air spaces (Fig. 19-4C) (Groniowski and Byczyskowa, 1964; Askin and Kuhn, 1971). Surfactant accumulates in the cytoplasm of pneumocytes type II in the form of characteristic, large osmiophilic lamellar inclusions that can be demonstrated by electron microscopy (Fig.19-4D). The precursor proteins of surfactant and the lamellar inclusions are markers of pneumocytes type II. As noted, these cells can regenerate if injured, and are also capable of differentiating into pneumocytes type I (Kasper and Haroske, 1996).
Pneumocytes are not likely to be recognized in specimens of sputum or bronchial brushing, but they can be identified in bronchoalveolar lavage (BAL) and fine-needle aspiration (FNA) specimens of lung from patients with chronic lung disease and may be mistaken for adenocarcinoma (see below).
Pulmonary Alveolar Macrophages
In histologic material that has been handled carefully and processed without excessive delay, large phagocytic cells containing particles of dust are observed within nearly all of the alveoli (Fig. 19-5). These cells are sometimes referred to as dust cells or pneumocytes type III. Exceedingly large numbers of macrophages may be observed in the alveoli of
people who are heavy cigarette smokers or live in a dusty atmosphere. The ultrastructure of alveolar macrophages is consistent with a metabolically active cell provided with microvilli, lysosomes, and vacuolated inclusions. The bone marrow origin of alveolar macrophages was demonstrated in mice by Brunstetter et al (1971), and in humans by Thomas et al (1976) and Nakata et al (1999) who used the FISH (fluorescent in situ hybridization) technique (see Chaps. 3 and 4) to demonstrate a Y chromosome in the alveolar macrophages of a female recipient of bone marrow from a male donor. The phagocytic function of alveolar macrophages



people who are heavy cigarette smokers or live in a dusty atmosphere. The ultrastructure of alveolar macrophages is consistent with a metabolically active cell provided with microvilli, lysosomes, and vacuolated inclusions. The bone marrow origin of alveolar macrophages was demonstrated in mice by Brunstetter et al (1971), and in humans by Thomas et al (1976) and Nakata et al (1999) who used the FISH (fluorescent in situ hybridization) technique (see Chaps. 3 and 4) to demonstrate a Y chromosome in the alveolar macrophages of a female recipient of bone marrow from a male donor. The phagocytic function of alveolar macrophages
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree