The Liver and Spleen
Muhammad B. Zaman
THE LIVER
Diseases of the liver can be broadly divided into diffuse parenchymal disorders, such as hepatitis and cirrhosis, and space-occupying lesions, such as cysts, abscesses, and benign or malignant tumors. The latter group is the target of fine-needle aspiration biopsy (FNA), which is performed under imaging guidance [ultrasonography (US) or computed tomography (CT)], usually by an interventional radiologist. Lundquist (1971) attributed the actual introduction of the aspiration of the liver to Lucatello in 1895.
At most institutions, including ours, the liver is the most commonly aspirated abdominal organ, accounting for 55% of abdominal aspirates and 36% of all aspirates performed by radiologists.
SAMPLING TECHNIQUES
CT and US can be used to guide the selection of the entry site for a liver aspiration biopsy, and provide information on the depth to which the needle will be inserted and the optimum angle of approach to the target. The entry site is selected based on the shortest distance between the skin and the target lesion. This entry site may be modified in order to avoid the costophrenic angles (and hence the danger of pneumothorax) and major vessels, such as the aorta, vena cava, and portal veins.
The patient is placed in a comfortable supine position, usually leaning to the left. Following the selection of the entry site, the skin is cleansed and anesthetized. A 2- to 3-mm skin incision made with a no. 11 scalpel blade facilitates the passage of the biopsy needle. The needle, with the stylet in place, is inserted through the skin incision at the previously determined angle and depth. The insertion of the needle is performed during suspended respiration and usually in the same respiratory phase employed in the previous localizing study. Special care and experience are required to prevent the direction of the thin, flexible needle from being deflected, particularly in patients with well-developed musculature. This problem can be avoided by initially inserting a rigid 18-gauge needle with a stylet to the depth of subcutaneous fat and muscle. Subsequently the stylet is removed and replaced by the thin needle, which is then advanced to the target. As the needle reaches the target, the operator is often able to sense a change in the consistency of the tissue. Five to 10 rapid (4- to 5-mm) excursions of
the needle are performed to loosen the cells. Suction is applied by attaching a 10- or 20-ml disposable syringe to the thin needle. This is followed by a few short excursions through the lesion, while the suction is continued. The suction must be released before the needle is withdrawn. Except for cysts with a large fluid content, the aspirated material should remain within the needle. The operator can repeat this procedure three to four times through the same skin incision, using a different needle and slightly modifying the angle of approach.
the needle are performed to loosen the cells. Suction is applied by attaching a 10- or 20-ml disposable syringe to the thin needle. This is followed by a few short excursions through the lesion, while the suction is continued. The suction must be released before the needle is withdrawn. Except for cysts with a large fluid content, the aspirated material should remain within the needle. The operator can repeat this procedure three to four times through the same skin incision, using a different needle and slightly modifying the angle of approach.
Recently there have been further innovations in the technique. An 18-gauge spring-driven biopsy gun (Automatic Disposable Guillotine Soft-Tissue Needle; Bauer Medical Inc, Clearwater, FL), similar to the device extensively used for a Tru-Cut prostate needle biopsy, is employed. A 15-cm-long, 1.5-mm-wide outer needle with a stylus is first introduced under CT guidance to reach the lesion. The longer (20 cm), precocked, 18-gauge biopsy gun is then introduced to the appropriate depth and multiple slender tissue cores are obtained for cytologic and histologic studies. An immediate assessment of a core can be performed with smears, prepared as crush or touch preparations, stained with Diff-Quik. Two to four smears from each biopsy can be prepared. It was previously documented by Sherlock et al (1967), Grossman et al (1972), and Carney (1975) that the cytologic evaluation of residual debris accompanying large core needle biopsies increases the diagnostic yield in malignant diseases in comparison with histologic evaluation. In our experience, in 5% to 10% of cases of metastatic cancer, only benign liver tissue is seen in routine histologic sections of hepatic biopsies (cut at six to eight levels), whereas smears disclose malignant cells. Thus, cytologic and histologic studies of liver biopsies are complementary and the use of both methods can increase the diagnostic sensitivity (Bell, 1986; Dusenbery et al, 1995). The reported sensitivity of tumor diagnoses ranges from 67% to 100%, and the specificity ranges from 80% to 100% (Schwerk et al, 1981; Walker et al, 1982; Samartonga et al, 1992; Hertz et al, 2000).
Complications
FNA of the liver is considered a very safe procedure. Anecdotal cases of fatality secondary to hemorrhage have been reported (Riska et al, 1975), and bleeding disorders are considered a contraindication. The other reported complication is bile peritonitis (Schultz, 1976). At Montefiore Medical Center, we observed a similar case occurring in a patient with pancreatic carcinoma and distended gall bladder (Courvoisier gall bladder), and a case of fatal hemorrhage following aspiration of a hepatic angiosarcoma (Rosenblatt et al, 1982; Koss et al, 1992). DeMay (1996) recorded several cases of seeding of primary and metastatic malignant tumors of the liver after these diagnostic procedures were performed. In some of these procedures, large-caliber needles were used.
TABLE 38-1 PRINCIPAL SPACE-OCCUPYING LESIONS OF THE LIVER | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||
Indications
FNA of the liver is widely used in the evaluation of space-occupying lesions. The principal targets are listed in Table 38-1. In metastatic cancer, the superiority of the guided needle approach over the transcutaneous tissue core biopsy performed with large-bore needles (Vim-Silverman- or Menghini-type needles) has been documented, particularly for lesions in the left lobe of the liver (Rosenblatt et al, 1982).
As discussed below, FNA of the liver does not replace tissue biopsy for the evaluation of diffuse hepatic disorders such as cirrhosis, hepatitis, suspected granulomas, or metabolic diseases, in which the histologic context is important. However, an incidental cytologic examination may sometimes contribute to the diagnosis in such cases.
NORMAL LIVER
Histology
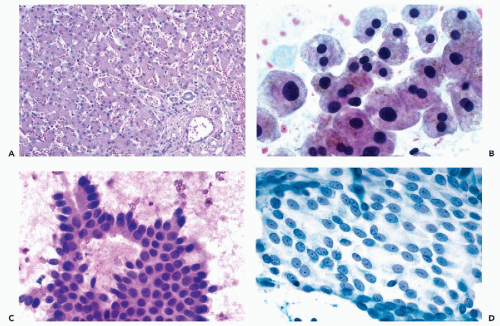
The liver is enclosed in a thin layer of connective tissue surfaced by a single layer of mesothelial cells (Glisson’s capsule), and is separated from the right diaphragm by a narrow space. Normal liver is composed of numerous lobules, each of which is formed by large hepatocytes arranged in plates that are separated from each other by capillary vessels (Fig. 38-1A). The endothelial cells of these capillaries, the Kupffer cells, have a phagocytic function. Rare, small perisinusoidal cells, hepatic stellate cells (Ito cell or fat-storing cell), become prominent in hypervitaminosis
A. The hepatic lobules are separated from each other by portal spaces, or areas of connective tissue, that contain small bile ducts and branches of the portal vein and hepatic arteries.
A. The hepatic lobules are separated from each other by portal spaces, or areas of connective tissue, that contain small bile ducts and branches of the portal vein and hepatic arteries.
A characteristic feature of the metabolically very active hepatocytes is the granularity of their cytoplasm, which is caused by the presence of numerous organelles (mainly mitochondria). Most, but not all, nuclei of normal adult hepatocytes are tetraploid, which accounts for their relatively large sizes.
One of the principal functions of hepatocytes is the formation of bile, which is collected in tiny intercellular bile canaliculi. These canaliculi merge to form intralobular bile ducts that may be visualized by special stains, such as CK19 (Hurliman et al, 1991; Maeda et al, 1995, 1996; Alexander et al, 1997). The intralobular ducts merge to form larger bile ducts that are visible in portal spaces and eventually deliver the bile into the common bile duct, which opens into the duodenum. The smaller bile ducts are lined by cuboidal cells, and the larger ducts are lined by columnar cells with clear cytoplasm and small vesicular nuclei.
Cytology
Normal liver is never deliberately aspirated, but normal hepatocytes and bile duct cells are a common component of smears, particularly if the target of the aspiration is a relatively small space-occupying lesion. Mesothelial cells, which are derived from the mesothelial lining of Glisson’s capsule (see below), are a common component of such smears.
Normal and Reactive Hepatocytes
Hepatocytes occur singly or form loosely cohesive groups of large, flat polygonal cells with abundant, dense granular cytoplasm that stains pink with hematoxylin and eosin, and purple with hematologic stains (such as Diff-Quik) or orange-brown with Papanicolaou stain (Fig. 38-1B). Quite often, the cytoplasm is frayed at the edge. Perinuclear accumulation of lipofuscin pigment may be present in older patients (see below). The single or double nuclei of hepatocytes are spherical, have a regular contour, are centrally placed within the cell, and may vary in size in keeping
with their DNA content. The chromatin is moderately granular and evenly distributed, and small nucleoli are visible. Intranuclear cytoplasmic inclusions (INCIs) are not uncommon. Normal hepatocytes show some variability in size, and thus some degree of pleomorphism. However, a low nucleocytoplasmic ratio is maintained (i.e., smaller cells have smaller nuclei). In rapidly fixed material, hepatocytes in clusters may be separated from each other by narrow clear spaces that represent intercellular bile canaliculi. Kupffer cells are rarely identified unless they act as macrophages and contain phagocytized material in their cytoplasm.
with their DNA content. The chromatin is moderately granular and evenly distributed, and small nucleoli are visible. Intranuclear cytoplasmic inclusions (INCIs) are not uncommon. Normal hepatocytes show some variability in size, and thus some degree of pleomorphism. However, a low nucleocytoplasmic ratio is maintained (i.e., smaller cells have smaller nuclei). In rapidly fixed material, hepatocytes in clusters may be separated from each other by narrow clear spaces that represent intercellular bile canaliculi. Kupffer cells are rarely identified unless they act as macrophages and contain phagocytized material in their cytoplasm.
Bile Duct Epithelium
Bile duct cells usually form flat, cohesive clusters with distinguishable cell borders (i.e., a honeycomb arrangement of cells). Cells derived from small bile ducts are cuboidal, whereas larger columnar cells, similar to the endocervical epithelium, are derived from larger ducts. In both types of cells, the nuclei are transparent, uniform, ovoid, and basally placed (Fig. 38-1C). In normal liver aspirates, bile duct cell clusters are relatively uncommon. The only situations in which they may represent the dominant population in an FNA smear are those involving a bile duct adenoma (peribiliary gland hamartoma) and bile duct hamartoma (see Fig. 38-17). Conversely, a total absence of bile duct epithelium in an adequate FNA of liver with uniform, relatively small hepatocytes should raise the suspicion of liver cell adenoma or a well-differentiated hepatocellular carcinoma (HCC).
Mesothelial Cells
During the aspiration, the needle must penetrate the serosal surface of the abdominal cavity and Glisson’s capsule before it reaches the target. Therefore, if the needle stylet is withdrawn before the needle enters the liver, sheets of mesothelial cells with characteristic intercellular clear spaces or “windows” may be observed. A characteristic feature of these cells is the presence of visible, sometimes multiple nucleoli within the oval or spherical, granular nuclei. In some liver smears, particularly if the aim of the aspiration is a small subcapsular lesion (e.g., a hemangioma), only mesothelial cells may be present. If such smears are inexpertly prepared, the sheets of mesothelial cells may be disorganized. Because of their nuclear features, the mesothelial cells may be mistaken for cancer cells (Fig. 38-1D).
Hemopoietic cells are seen in extramedullary hematopoiesis, which may occur normally in infants but in adults is a consequence of impaired bone marrow function. Large, multilobate megakaryocytes are usually the most conspicuous cells in such smears (see Fig. 40-22).
PIGMENTS IN LIVER CELLS
Lipofuscin
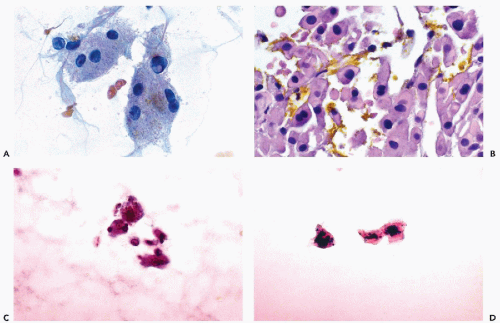
This very common pigment, which is located predominantly within the perinuclear area of centrilobular hepatocytes, forms fine nonrefractile brown to golden-brown granules in Papanicolaou-stained smears (Fig. 38-2A) The pigment is composed of tertiary lysosomes that accumulate as end-products of intracellular digestion, and the amount of pigment increases with age. Lipofuscin can be stained with acid-fast stain (Fite stain), and has no particular clinical significance; however, it may be confused with other pigments that may accumulate in pathologic states.
Bile
Intracellular bile is a nonrefractile pigment that stains green to greenish-brown in Papanicolaou stain, and dark blue in Diff-Quik stain. Bile is produced only by hepatocytes, and in well-fixed smears the pigment is observed in tiny intercellular bile canaliculi. Otherwise, the pigment is diffuse in the cytoplasm. Accumulation of bile in benign hepatocytes is associated with hepatitis or obstructive jaundice. In the latter condition, bile may also be observed as yellow-green extracellular crystals (Fig. 38-2B). The presence of bile in the cytoplasm of malignant cells is diagnostic of primary or metastatic HCC.
Hemosiderin
Hemosiderin is a coarse, golden-brown refractile pigment that can be seen in the cytoplasm of hepatocytes, Kupffer cells, and, rarely, in bile duct epithelium. Its identity can be confirmed by stains for iron, such as Prussian blue. Its presence in hepatocytes indicates iron overload that may lead to cirrhosis. This may occur in an iron metabolism disorder (e.g., hemochromatosis) or after numerous transfusions (e.g., hemosiderosis) (Fig. 38-2C,D).
Melanin
This pigment is usually associated with metastatic melanoma to the liver. This is a powdery cytoplasmic pigment that stains brown in Papanicolaou stain (see Fig. 38-23A) and blue in Diff-Quik stain. The pigment may also be phagocytized by Kupffer cells. For further discussion of metastatic malignant melanoma, see below.
Copper
This coarse, green-brown pigment is seen in a perinuclear cytoplasmic location in the hepatocytes of patients with copper metabolism disorders, such as Wilson’s disease, Indian childhood cirrhosis (possibly caused by excessive dietary intake of copper resulting from the use of copper or brass cooking and storage vessels), primary biliary cirrhosis, and other chronic cholestatic disease (Sipponen et al, 1980). Special stains are necessary to identify copper.
DIFFUSE DISEASES OF THE LIVER
Limitations of FNA
As mentioned in the introductory comments of this chapter, aspiration biopsy smears are generally inadequate for the
diagnosis of diffuse or medical diseases of the liver, which require the use of tissue biopsies, preferably representing six to 12 portal tracts. However, occasionally aspiration smears combined with other laboratory data may contribute to the differential diagnosis of these disorders, particularly in ruling out suspected HCC. The two principal disorders in this category are cirrhosis and chronic hepatitis.
diagnosis of diffuse or medical diseases of the liver, which require the use of tissue biopsies, preferably representing six to 12 portal tracts. However, occasionally aspiration smears combined with other laboratory data may contribute to the differential diagnosis of these disorders, particularly in ruling out suspected HCC. The two principal disorders in this category are cirrhosis and chronic hepatitis.
Cirrhosis
Histology
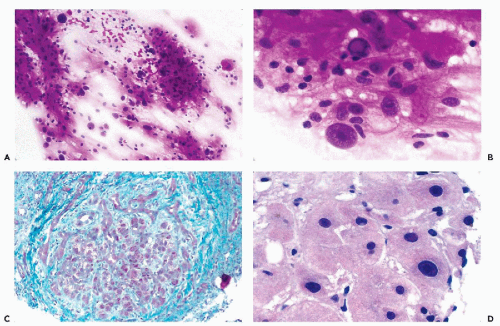
Cirrhosis, which is an essentially irreversible process, may be the end stage of alcoholic liver disease or of chronic hepatitis caused by hepatitis virus B or C. Excessive growth of portal connective tissue, with a resulting subdivision of the liver into visible and palpable coarse nodules of various sizes, is the essence of the cirrhotic process (Fig. 38-3C). Within the nodules, necrosis and regeneration of hepatocytes may take place, and this process is thought to be involved in the pathogenesis of HCCs that may complicate cirrhosis. Within the enlarged portal spaces, there is usually a marked proliferation of bile ducts and an inflammatory infiltrate composed mainly of lymphocytes. Because the pathologic subdivision of the liver parenchyma disturbs the normal circulation of blood with increased portal venous pressure, the various manifestations of cirrhosis include esophageal and periumbilical (caput medusae) vein enlargement or varices, enlargement of the spleen, and accumulation of ascitic fluid (Koss et al, 1992). Hemorrhages from the esophageal varicose veins may be fatal to the patient. In general, cirrhotic livers are not investigated by FNA. However, on CT scans cirrhotic nodules may mimic HCC, and this may lead to an aspiration.
Cytology
The cellular and nuclear pleomorphism of hepatocytes with many multinucleated cells (some with intranuclear cytoplasmic inclusions) is the hallmark of cirrhosis (Fig. 38-3A,B). The nuclei may also vary in size, and some may show prominent nucleoli. However, the nucleocytoplasmic ratio is usually not altered (Fig. 38-3D). Fragments of fibrous tissue that incorporate pleomorphic hepatocytes or encircling hepatic nodules are occasionally present in smears (Fig. 38-3A) (Lundquist, 1970). Bile duct epithelium is usually well represented, a feature that is not seen in FNA of large HCC. The latter finding is very helpful because when the cellular pleomorphism is very marked, and particularly in the presence of occasional large, hyperchromatic nuclei with prominent nucleoli stripped of cytoplasm, the differential
diagnosis of cirrhosis from HCC may pose problems (Berman, 1988; Koss et al, 1992).
diagnosis of cirrhosis from HCC may pose problems (Berman, 1988; Koss et al, 1992).
Biliary Cirrhosis
Biliary cirrhosis is an uncommon autoimmune disorder that mainly affects women and leads to the destruction of bile ducts. A characteristic feature of this disorder is the presence of antimitochondrial antibodies (for recent reviews see Tanaka et al, 2001; Bogdanos et al, 2003; Zuber and Recktenwald, 2003). This disorder is rarely, if ever, dignosed by needle aspiration biopsy. It is mentioned here because occasionally an HCC will develop in such patients (Morimoto et al, 1999).
Chronic Hepatitis
The diagnosis of chronic hepatitis, particularly that caused by hepatitis virus C, requires the use of core biopsies, which are assessed by an elaborate grading and staging system (Ishak et al, 1995). An incidental FNA performed because of suspicion of HCC will reveal hepatocytes that vary in appearance from essentially normal to pleomorphic, as observed in cirrhosis (see above). The presence of scattered lymphocytes may suggest the correct diagnosis. Mummified, shrunken, eosinophilic, necrotic hepatocytes (Councilman or acidophilic bodies) may be seen.
Metabolic Disorders
Inborn hereditary errors of metabolism that diffusely involve the liver and are seen in pediatric-age groups are not a primary indication for an aspiration biopsy. The reader is referred to any standard textbook of pathology to review the natural history and microscopic findings in such disorders, which include alpha-1-antitrypsin deficiency, cystic fibrosis, glycogen storage disease type IV, mucopolysaccharidosis, porphyria cutanea tarda, and erythropoietic protoporphyria.
Some metabolic disorders are amenable to primary FNA biopsy diagnosis (e.g., amyloidosis (Bose, 1989) and sometimes Gaucher’s disease) (see Fig. 38-25). Others result in the deposition of pigments in the hepatocytes (e.g., hemachromatosis and Wilson’s disease), as discussed above.
Cytoplasmic vacuoles containing fat (fatty liver or steatosis) are attributed to faulty metabolism or alcoholic liver disease (Fig. 38-4A). Regenerating liver, following partial resection, may show enlarged nuclei with prominent
nucleoli and steatosis. Such changes are sometimes termed “reactive atypia,” and should be reported with an explanatory note (Fig. 38-4B).
nucleoli and steatosis. Such changes are sometimes termed “reactive atypia,” and should be reported with an explanatory note (Fig. 38-4B).
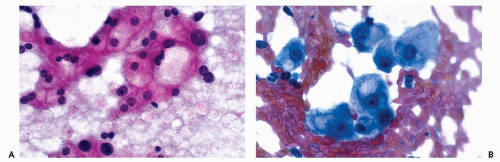 Figure 38-4 Metabolic disorders. A.
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|