aspiration (FNA) of the kidney. Dean (1939) first reported the treatment of a solitary cyst of kidney by aspiration. Söderström (1966) described a large experience with this method, and Von Schreeb et al (1967), Kristensen et al (1972), Thommesen and Nielsen (1975), Edgren et al (1975), and Holm et al (1975) each reported a series of cases.
Cystic lesions (as a diagnostic and therapeutic procedure)
Lesions with equivocal radiologic findings
Confirmation of diagnosis in advanced malignant lesions prior to nonsurgical treatment
Confirmation of local recurrence at the site of a prior tumor or a direct extension from neighboring site (e.g., colon or adrenal)
Confirmation of metastatic cancer
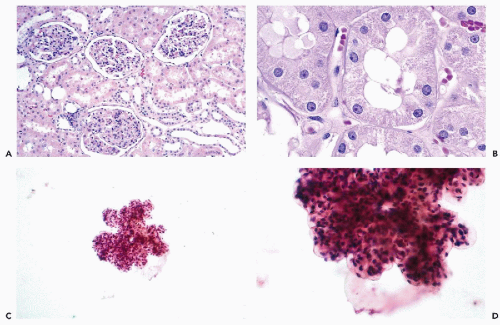
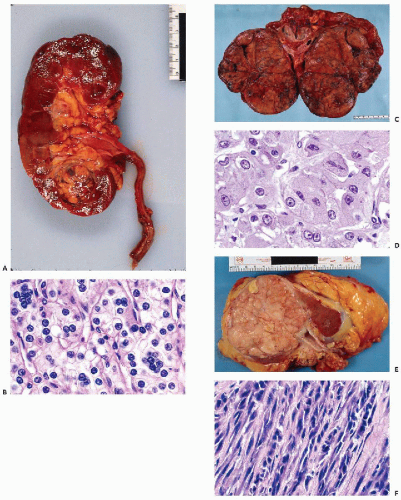
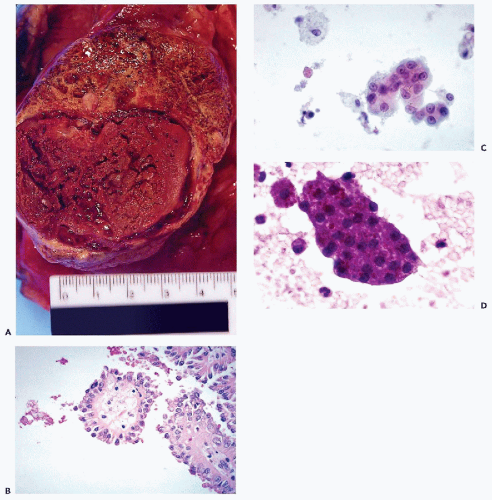
are often observed. However, unless a large-bore needle is used, intact glomeruli are rarely encountered (Fig. 40-1C,D).
yields bloody fluid with high fat and protein but low LDH levels.
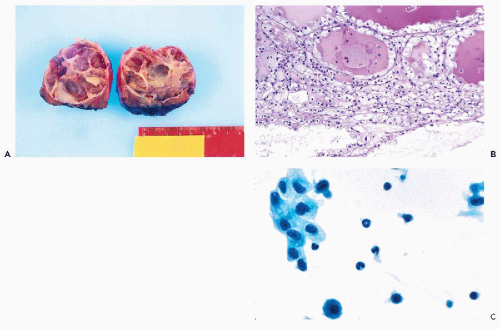
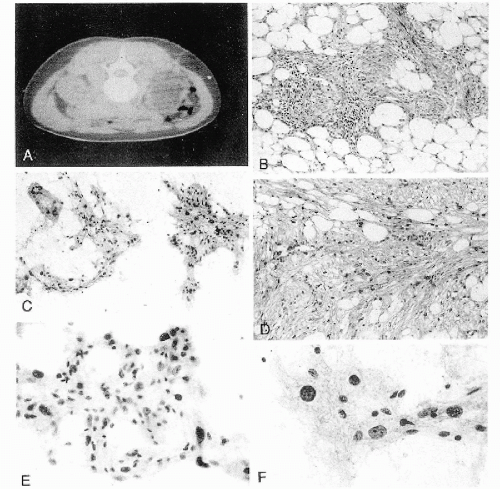 Figure 40-4 Angiomyolipoma. Angiomyolipoma of the left kidney in a 34-year-old woman with no evidence of tuberous sclerosis. A. Computed tomography (CT) revealed the tumor in the lower pole of the right kidney. The patient was in a prone position. The CT features were characteristic of angiomyolipoma, notably because of the presence of clear areas representing fat within the tumor. B,D. Tissue sections. B. Numerous thick-walled vessels, smooth muscle cells, and fat are present. D. Bundles of smooth muscle cells. Note the presence of pleomorphic, atypical nuclei. C,E,F. Aspirate. C. Irregular tissue fragments containing smooth muscle cells, fat, and vessels. E. Tissue fragment. Note the irregular arrangement of the nuclei and their variable sizes. F. Group of cells with abundant cytoplasm. Note the significant differences in nuclear sizes. Most of the nuclei are hyperchromatic, but their contour is smooth. These cytologic features may lead to an erroneous diagnosis of a carcinoma or a sarcoma. (From Koss et al. Aspiration Biopsy. Cytologic Interpretation and Histologic Bases, 2nd ed. New York: Igaku-Shoin, 1992.) |
TABLE 40-1 CLASSIFICATION OF RENAL CELL NEOPLASMS | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
related to papillary RCC, which shows similar findings (Brown et al, 1997). We recently encountered a case of papillary carcinoma that arose in a metanephric adenoma, confirming the relationship between the two lesions.
tumors invade beyond Gerota’s fascia, or are metastatic to distant organs. Metastases to regional lymph nodes upstages stage 1 and 2 tumors to stage 3 tumors.
TABLE 40-2 RENAL CELL NEOPLASMS: PRESUMED CELL OF ORIGIN AND CYTOGENETIC ABNORMALITIES* | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
TABLE 40-3 FUHRMAN’S GRADING OF CONVENTIONAL RENAL CELL CARCINOMA | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 40-4 MORPHOLOGIC VARIANTS OF CONVENTIONAL RCC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
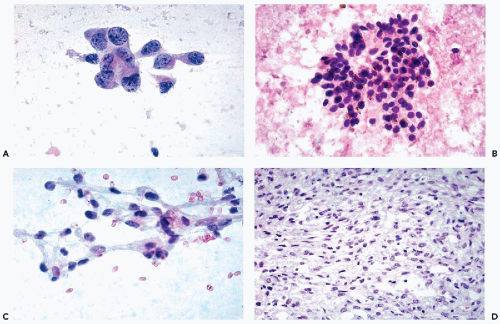
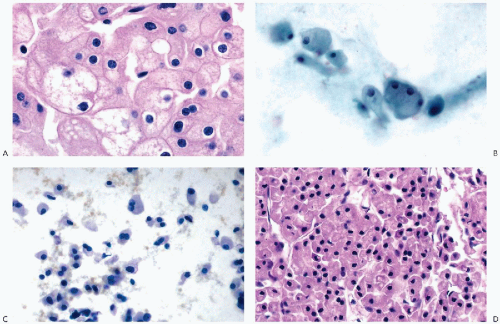
that is often filled with numerous small vacuoles containing lipid and glycogen but not mucin (Fig. 40-7B). The vacuoles are better seen in air-dried smears processed with hematologic stains. Phagocytosed hemosiderin may be seen. The nuclei are relatively small, but still much larger than those of benign tubular cells. They are only slightly pleomorphic, haphazardly placed, usually hyperchromatic, and contain readily visible nucleoli.
well-differentiated hepatomas was absent in RCC (see Chap. 38).
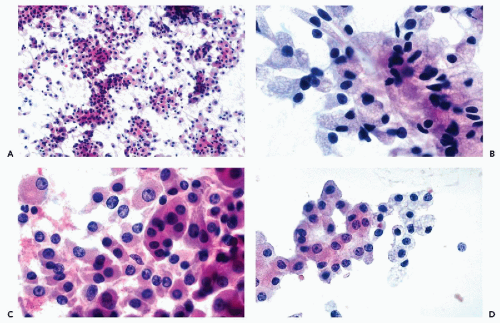
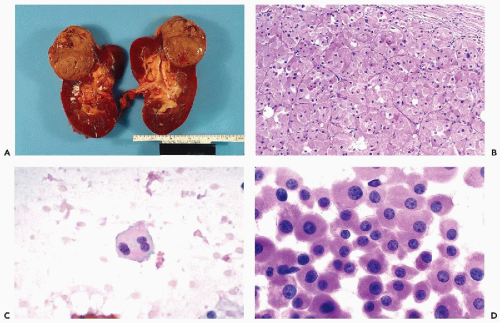
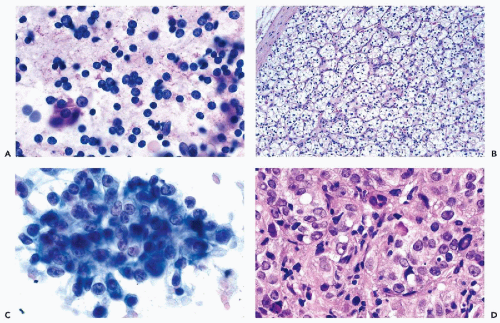 Figure 40-8 FNA of low- and high-grade renal carcinomas. A. FNA of a small RCC (shown in Fig. 40-6A) shows numerous well preserved “naked” nuclei, with some cells still retaining their eosinophilic granular cytoplasm. B. Histology of the same case shows a low-grade (grade 1) conventional RCC, clear cell type. C,D. Cells of a high-grade RCC with clear, scanty cytoplasm forming a cluster. B. Corresponding histology is from metastasis to the liver (core biopsy). |
TABLE 40-5 RENAL CELL TUMORS WITH GRANULAR CYTOPLASM | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
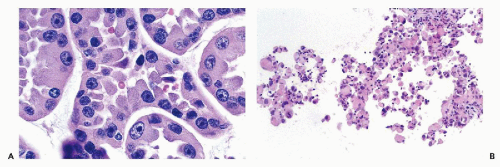
nucleoli of moderate sizes are often present. The neoplastic cells often contain large amounts of ingested hemosiderin (Fig. 40-10). Psammoma bodies are not uncommon, particularly in tumors with large cells. Solid variants of PRCC have been described based on cytogenetic findings (Renshaw et al, 1997).
in most cases is very pale and transparent (typical chromophobe tumors) or, in some cases, eosinophilic and granular (Fig. 40-12D).
Characteristically, the cells stain strongly with Hale’s colloidal iron, express epithelial membrane antigen (EMA), and are vimentin-negative (see Table 40-5).
of well-differentiated oncocytes with small nuclei, the prognosis is excellent and the tumor is considered to be benign. However, there have been reports that at least some tumors may invade the parenchyma of the kidney and may recur (Rodriguez et al, 1980; Lieber et al, 1981). For tumors diagnosed as oncocytomas that produce metastases, the possibility of misclassification must be considered. As an example, we recently reviewed a case that was initially classified as oncocytoma and was resected in 1985. Two years later, a mass in the liver was discovered and aspirated. The FNA smears strongly suggested that the cells represented metastatic renal tumor. The “oncocytoma” from 1985 was reclassified as ChRCC (Fig. 40-12A,B). Also, oncocytomas may be associated with conventional RCC (Klein and Valensi, 1976; Fromowitz and Bard, 1990).
of which have granular cytoplasm (Fig. 40-14A), and of nests of clear cells embedded in fibroblastic stroma that is sometimes very dense. Mucin may be present in some of the glandular structures. Adjacent renal tubules may show dysplastic changes (Kennedy et al, 1990), and psammoma bodies may be present.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree