Chapter 12 THE KIDNEYS
Introduction
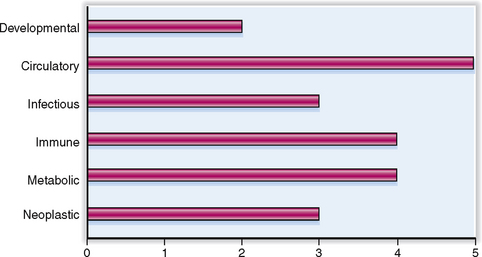
The kidneys are part of the urinary system (tract). The clinical significance of urinary tract diseases may be best illustrated by the fact that urinary tract infections (UTIs) are among the most common infections encountered in general medical practice. Between 1% and 2% of all visits to doctors’ offices are prompted by UTIs. One in 5 women between the ages of 20 and 45 years has periodic problems with urination, and 5% of all women in that age group experience a UTI. The rate of infection rises to 15% by the age of 65 years. Urinary tract tumors account for 10% of all malignant tumors in men and 4% in women. The relative clinical significance of various renal diseases is presented graphically in Figure 12-1.
 Acute tubular necrosis with significant retention of metabolic by-product and waste material occurs in most patients who have undergone prolonged major surgery, and in many patients who have been in shock or have suffered major trauma.
Acute tubular necrosis with significant retention of metabolic by-product and waste material occurs in most patients who have undergone prolonged major surgery, and in many patients who have been in shock or have suffered major trauma.
 Renal dysfunction is encountered in the course of many systemic diseases, such as diabetes mellitus, arterial hypertension, or systemic lupus erythematosus (SLE).
Renal dysfunction is encountered in the course of many systemic diseases, such as diabetes mellitus, arterial hypertension, or systemic lupus erythematosus (SLE).
 Renal failure may develop due to a failure of another organ, such a prerenal failure due to congestive heart disease or hepatorenal syndrome as a complication of cirrhosis.
Renal failure may develop due to a failure of another organ, such a prerenal failure due to congestive heart disease or hepatorenal syndrome as a complication of cirrhosis.
 The overall incidence of chronic renal disease is approximately 150 per million persons, and most of these patients require long-term dialysis or renal transplantation.
The overall incidence of chronic renal disease is approximately 150 per million persons, and most of these patients require long-term dialysis or renal transplantation.
Normal Anatomy and Physiology
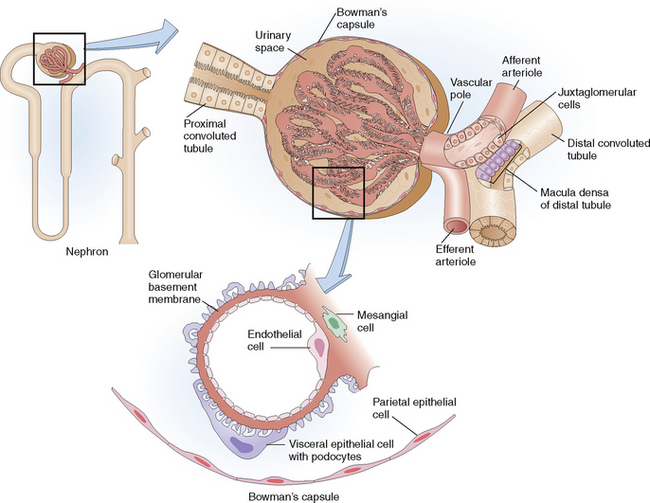
Afferent arteriole Small branch of the interlobular renal artery that enters the glomerulus on its vascular pole. It brings arterial blood into the glomerulus. The afferent arteriole branches into glomerular capillaries, which by confluence form the efferent arteriole. Blood leaves the glomerulus through the efferent arteriole, which branches into peritubular capillaries or vasa recta. At its entry into the glomerulus the afferent arteriole wall contains juxtaglomerular cells.
Blood supply The kidneys receive 20% to 25% of the cardiac output. Approximately 90% of the arterial blood remains in the cortex, 9% enters the outer medulla, and only 1% reaches the inner medulla. Kidneys maintain a constant blood flow by autoregulation that functions when the perfusion pressure is in the range from 60 to 180 mm Hg.
Glomerular basement membrane (GBM) Selectively permeable specialized vascular basement membrane outlining the glomerular capillaries. It regulates the ultrafiltration of plasma and the formation of the primary urinary filtrate. Together with the fenestrated endothelial cells and the foot processes forming epithelial cells (podocytes), the GBM forms the glomerular capillary wall (GCW).
Glomerular filtration rate (GFR) Rate at which the plasma is filtered in the glomeruli to form the glomerular urinary filtrate. The GFR can be expressed as milliliters of fluid per minute or liters per day. Since the renal plasma flow is 600 mL/min and the ratio of renal plasma flow to GFR is constant at 5:1, the GFR is approximately 120 mL/min.
Glomerular tuft Convolution of fenestrated capillaries that have a highly specialized membrane designed for selective ultrafiltration of plasma. The glomerular tuft contains three cell types: the endothelial, epithelial, and mesangial cells.
Glomerulus Initial portion of the nephron interposed between the afferent and efferent arterioles on one side and the proximal convoluted tubule on the other. It is composed of a tuft of fenestrated capillaries enclosed by Bowman’s capsule.
Juxtaglomerular (JG) apparatus Structure composed of specialized renin-secreting cells localized in the wall of the afferent arteriole at the vascular pole of the glomerulus, along with the cells of macula densa a specialized part of the distal convoluted tubule.
Kidneys Paired organs located in the retroperitoneum, connected on one side to renal arteries and veins and on the other to the ureter. On cross section, the kidney has two distinct parts: an outer part called the cortex and an inner one called the medulla. The main function of the kidney is the formation of urine and elimination of superfluous water and minerals, metabolic waste products, drugs, and xenobiotics. The kidneys secrete some hormones and growth factors, such as renin and erythropoietin.
Mesangium Connective tissue framework of the glomerulus extending into the glomerular tuft from the vascular pole. It consists of mesangial cells and nonfibrillar extracellular matrix that fills the space delimited by the confluence of three to four glomerular capillaries.
Nephron Basic functional unit of the kidney comprising the glomerulus, the proximal convoluted tubule, the loop of Henle, the distal tubule, and the collecting ducts. On one side the nephron is linked to the arterial blood supply and on the other with the excretory urinary ducts. There are two types of nephrons: cortical nephrons (which have short loops of Henle) and juxtamedullary nephrons (which have long loops of Henle extending into the medulla). The former are surrounded by capillary networks and the latter by vasa recta, the only blood supply for the deep medulla and the papillae.
Renal arteries There are two renal arteries both of which originate from the abdominal aorta. In the kidneys they branch, forming large interlobar arteries, which in turn give rise to arcuate arteries running along the corticomedullary junction. The arcuate arteries give rise to the cortical interlobar arteries, which give rise to the afferent arterioles. The efferent arteriole exiting the glomerulus gives rise to peritubular capillaries and vasa recta, which drain into the small renal veins.
Renin Enzyme secreted by the juxtaglomerular cells in response to reduced blood supply to the kidneys. It acts on angiotensinogen, transforming it into angiotensin, which stimulates aldosterone production in the adrenal cortex. Through this mechanism renin raises the blood pressure.
Ureter Tubular organ serving as the conduit for urine from the renal pelvis to the urinary bladder.
Urethra Tubular organ serving as the final conduit for the fluid exiting the urinary bladder during micturition. In males it also serves as a passageway for semen.
Urinary bladder Hollow organ whose primary function is to store urine prior to micturition.
Urine Fluid formed in the kidney from ultrafiltered plasma, containing, in addition to water, minerals, organic waste material, and possibly some xenobiotics. Urine is formed in the nephron through a stepwise process that includes ultrafiltration of the plasma, selective and site-specific reabsorption of some minerals and water, and secretion of others.
Urothelium (transitional epithelium) Specialized epithelium lining the renal calices, pelvis, ureters, urinary bladder, and the posterior part of the urethra.
Vitamin D Lipid-soluble vitamin hydroxylated in the liver and thereafter in the kidney, where the active form of the vitamin, 1,25(OH)2D3, is formed. With parathyroid hormone it regulates the absorption and metabolism of calcium.
Pathophysiology
Anuria Absence of urine, or in practical terms less than 100 mL of urine per day. Anuria is a sign of renal failure and may be classified as prerenal (e.g., due to reduced renal perfusion in heart failure), intrarenal (e.g., due to glomerulonephritis or toxic tubular necrosis), or postrenal (e.g., due to the obstruction of the ureters or bladder outflow tract).
Azotemia Increased blood concentration of nitrogen-containing compounds, such as urea or creatinine. The blood concentration of urea is conventionally expressed as blood urea nitrogen (BUN). Azotemia is usually a sign of renal failure, which may be prerenal, intrarenal, or postrenal.
Glycosuria Appearance of glucose in the urine in excess of the minimal amounts normally found in the urine (<200 mg/24 hours). It is a consequence of hyperglycemia, most often owing to diabetes mellitus.
Hematuria Appearance of blood in the urine. It may be macroscopic and visible to the naked eye or microscopic and detectable only by microscopic examination of the renal sediment.
Lipiduria Appearance of lipid droplets or lipid casts in the urine. Typically found in nephrotic syndrome and hyperlipemic states.
Oliguria Production of urine in small amounts. Typically it is a sign of renal failure, which may be prerenal, intrarenal, or postrenal. In adults oliguria is generally diagnosed when the total urinary output is below 400 mL/day.
Polyuria Production of large quantities of urine, reflected in both an increased frequency of urination (pollakisuria) and an abnormally large volume of fluid excreted over a 24-hour period. Empirically it is diagnosed in adults when the urine production exceeds 3 to 5 L/day.
Proteinuria Excretion of proteins in the urine in excess of the normal amount for the age of the patient. In healthy adults urine contains less than 500 mg of protein in 24 hours, but in children the urine contains less protein. Proteinuria is usually a sign of glomerular injury and occurs in nephritic and nephrotic syndromes. Bence Jones proteinuria is a feature of multiple myeloma and is characterized by urinary excretion of immunoglobulin light chains.
Pyuria Excretion of pus in the urine. Typically it is caused by bacterial infection.
Urinary casts Cylindrical structures found in the urine, typically formed from protein-rich contents of the renal tubules. Hyaline casts are normal components of urine and are formed predominantly from Tamm-Horsfall protein, which is secreted into the lumen of the distal tubules. Red blood cell casts contain deformed or fragmented red blood cells and are a sign of glomerular hematuria. Granular casts are composed of granules formed from disintegrated cells in tubular necrosis. White blood cell casts contain fragmented neutrophils and are found in inflammatory conditions.
Renal Diseases
Acute glomerulonephritis Inflammatory glomerular disease mediated by immune complexes. Immune complexes may be formed in situ in the glomeruli or may be deposited in the glomeruli from the circulation. Immune complexes can be formed between immunoglobulins and bacterial antigens, autoantigens, or glomerular antigens. The disease is characterized by an infiltrate of neutrophils or macrophages and proliferation of mesangial cells. Clinically it presents in the form of a nephritic syndrome.
Acute tubular necrosis Acute renal failure caused by necrosis of the renal tubular cells. It may result from ischemia (prerenal renal failure) or the action of toxins. Clinically it manifests with a loss of renal function. Typically the tubules cannot form urine or concentrate the primary filtrate. It manifests first with oliguria, followed by polyuria. In most instances tubular cells regenerate, and renal function is reestablished.
Acute tubulointerstitial nephritis Allergic reaction, most often precipitated by drugs. It is characterized by interstitial infiltrates of lymphocytes, eosinophils, and plasma cells and destruction of tubular cells. Clinically it manifests with acute renal failure.
Amyloidosis Systemic disease characterized by deposition of amyloid in many organs. It may be most often associated with the deposition of AA type amyloid, encountered in chronic inflammatory diseases, or AL amyloid, seen in multiple myeloma. Clinically it usually manifests with a nephrotic syndrome.
Crescentic glomerulonephritis Severe form of acute glomerulonephritis characterized by exudation of inflammatory cells into the pericapillary urinary space of the glomeruli, leading to the formation of crescents. It usually occurs after a focal necrotizing glomerulonephritis, typically caused by Goodpasture’s syndrome or Wegener’s granulomatosis, or less often after a severe form of acute postinfectious glomerulonephritis. Clinically it manifests as rapidly progressive glomerulonephritis (RPGN). Renal failure develops within 1 to 3 months of the onset of the disease.
Diabetic glomerulosclerosis Glomerular lesion induced by long-standing diabetes mellitus. Pathologically it has two forms: nodular glomerulosclerosis (Kimmelstiel-Wilson disease) with mesangial hyaline nodule formation, and diffuse glomerulosclerosis with diffuse thickening of the glomerular basement membranes. Both forms of glomerulosclerosis lead to proteinuria that may ultimately result in nephrotic syndrome, progressing to renal failure.
Focal glomerulosclerosis (FGS) Glomerular disease that may be idiopathic (primary) or secondary to so-called hyperperfusion injury of the glomeruli or another renal or systemic disease (e.g., AIDS). It is found in a subset of children who have nephrotic syndrome and are initially found to have minimal-change nephropathy (lipoid nephrosis). Morphologically it is recognized by partial obliteration of glomerular capillary tufts with hyaline material. Such changes are found only in some glomeruli, hence the name: focal, meaning some glomeruli, and segmental, meaning parts of the glomerular capillary tufts. Clinically it manifests as nephrotic syndrome unresponsive to treatment. Focal glomerulosclerosis is the most common cause of nephrotic syndrome in adults.
Glomerulonephritis Inflammation of the glomerulus, most often caused by immune mechanisms. It may be acute or chronic. Several morphologic variants of glomerulonephritis are found, such as necrotizing, proliferative, mesangial, crescentic, focal, and diffuse.
Goodpasture’s syndrome Renal–pulmonary syndrome mediated by cytotoxic antibodies to type IV collagen in glomerular and pulmonary capillary basement membranes. These antibodies cause focal and segmental necrotizing glomerulonephritis, which progresses to crescentic glomerulonephritis over a period of a month or two. Clinically it manifests as a RPGN with pulmonary hemorrhage.
Hydronephrosis Dilatation of the renal pelvis and calices with flattening of the renal papillae. If chronic hydronephrosis leads to an atrophy of the entire kidney parenchyma. It is typically caused by obstruction of the ureters with urinary stones, tumors, or extraureteral pathologic lesions (e.g., retroperitoneal fibrosis). The urine inside the hydronephrotic kidneys may become infected, leading to pyelonephritis or pyonephrosis (pus-filled hydronephrotic kidney).
Lupus nephritis Chronic glomerulonephritis, which occurs in approximately 75% of patients with systemic lupus erythematosus (SLE). It results from the deposition of circulating immune complexes in the glomeruli.
Membranous nephropathy Immune complex-mediated glomerular disease manifesting clinically as a nephrotic syndrome. Immune complexes are deposited on the epithelial side of the basement membranes and do not evoke inflammation.
Minimal-change disease Also know as “nil disease” or lipoid nephrosis, it is a disease of unknown origin. It manifests as nephrotic syndrome that usually responds well to treatment with steroids. Typically it occurs in children, but it may be found in adults as well. By light and immunofluorescence microscopy the glomeruli appear normal, and the only visible abnormality is the fusion of foot processes of the epithelial cells seen by electron microscopy.
Nephritic syndrome Clinical syndrome related to glomerulonephritis and characterized by oliguria, hematuria, proteinuria, hypoalbuminemia, and edema. Patients are typically hypertensive and have acute azotemia related to a reduced GFR. The urine is reduced in amount and dark brown. The urinary sediment contains red blood cell casts and dysmorphic and fragmented red blood cells. Most often it is a manifestation of postinfectious glomerulolonephritis and thus it heals in more than 90% of cases. In a minority of cases it follows a rapid downhill course, and in some cases it persists or progresses to chronic glomerulonephritis and end-stage renal failure.
Nephrosclerosis Term used to describe renal pathologic changes related to hyaline sclerosis of the arterioles and fibrosis of the intrarenal arteries. Benign nephrosclerosis is associated with hypertension and gradual loss of renal function leading to uremia. Accelerated nephrosclerosis, which shows fibrinoid necrosis of the arterioles and proliferative arteriolitis, is typically associated with malignant hypertension in African American men.
Nephrotic syndrome Clinical syndrome characterized by proteinuria, lipiduria, hypoalbuminemia, hyperlipidemia, and generalized edema. In children it is most often caused by minimal-change disease. In adults it is most often caused by focal glomerulosclerosis, but it may be due to membranous nephropathy, diabetic glomerulosclerosis, amyloidosis, or systemic lupus erythematosus (membranous type, class IV).
Pyelonephritis Bacterial inflammation of the kidney, which may be acute or chronic. Infection may reach the kidney upstream through urine backflow from the urinary bladder (ascending infection) or hematogenously (descending infection). Most often it is caused by uropathogens (i.e., E. coli, Proteus, Klebsiella, Enterococcus, Seratia sp.). The complications of acute pyelonephritis include papillary necrosis, pyonephrosis and perinephric abscess, or systemic sepsis (“urosepsis”). The most important complications of chronic pyelonephritis are arterial hypertension and, in bilateral cases, renal failure (uremia).
Rapidly progressive glomerulonephritis (RPGN) Term used for any form of renal failure of sudden onset associated with severe glomerular injury. Pathologically it manifests with crescentic glomerulonephritis. It is typical of anti-GBM cytotoxic antibody-mediated glomerulonephritis (Goodpasture’s syndrome) and pauci-immune glomerulonephritis (e.g., Wegener’s granulomatosis). It is lethal if not treated, but if immunosuppressive treatment is given in time, the patient may recover.
Renal cell carcinoma The most common malignant tumor of the kidneys. It originates from cells of the proximal tubules. Most often the tumor manifests with hematuria (50%) or nonspecific symptoms. It is thus called “internist’s tumor” because it is usually diagnosed by internists rather than urologists. It metastasizes through the renal veins to the lungs or locally to the lymph nodes.
Renal failure Loss of renal function, which may be classified as prerenal, intrarenal, or postrenal; acute or chronic. The most common cause of acute renal failure is prerenal renal failure, related to hypoperfusion of the kidneys due to low blood pressure. It occurs in heart failure, shock, hypotension after surgery, or trauma. Intrarenal renal failure is caused by primary glomerular or tubulointerstitial diseases. Postrenal renal failure is caused by urinary tract obstruction. Acute renal failure is usually reversible. Chronic renal failure is irreversible. It may be mild or severe (uremia).
Transitional cell carcinoma Malignant tumor originating from the renal calices and pelves. Histologically it is identical to urothelial carcinoma of the urinary bladder. It is usually papillary but may also be flat or invasive. Grade I tumors have an excellent prognosis but tend to recur. Grade III tumors have poor prognosis.
Tubulointerstitial nephritis Group of renal diseases affecting tubules and interstitium and clinically manifesting as reduced renal function. It may be related to an adverse reaction to drugs, abuse of analgesics, urate deposition, hypercalcemia, or multiple myeloma. Bacterial infections of the kidney are by convention not included under this heading but are called pyelonephritis.
Uremia Synonym for end-stage kidney failure. In this state the body cannot excrete the urinary waste products. Retention of these waste products results in azotemia (elevation of BUN and creatinine), metabolic acidosis, and dysfunction of many vital organs. Without renal dialysis or renal transplantation it is invariably lethal.
Urolithiasis Synonym for urinary stone disease. Urinary stones (uroliths) are chemically classified as (1) calcium oxalate/phosphate, (2) uric acid, (3) ammonium magnesium calcium phosphate (“struvite”), or (4) cysteine stones. Stones may form in the renal pelvis, ureters, or urinary bladder. Clinically most often they manifest with renal colic related to the impaction of stones in the ureters. Chronic obstruction leads to hydronephrosis and loss of renal function. Urinary stones also predispose to urinary infection.
Urinalysis is performed during the routine yearly medical check-up, for health employment, for insurance purposes, and essentially for all hospitalized patients. It provides a rough insight into the function of the kidneys and the urinary tract, but it also may give some indication about a person’s general health status. A more complex assessment of renal function requires additional testing and a higher degree of expertise, and therefore a consultation with a nephrologist is one of the most often required consultations in most hospitals.
Normal Structure and Function
NORMAL ANATOMY AND HISTOLOGY
The nephron is the basic functional unit of each kidney.
Each kidney is composed of approximately 1 million nephrons. Nephrons form the basic functional units and are composed of several components: the glomerulus, proximal convoluted tubule, loop of Henle, distal convoluted tubule, and collecting ducts (Fig. 12-2). The main function of the nephrons is the formation of urine, which begins by the filtration of the plasma in the glomeruli, followed by reabsorption of some components of the filtrate, secretion of additional substances, and final concentration of the filtrate into urine.
 Endothelial cells, the fenestrated capillaries lining the inside of the capillaries.
Endothelial cells, the fenestrated capillaries lining the inside of the capillaries.
 Glomerular basement membrane (GBM), the semipermeable membrane composed of collagen type IV, laminin, and some other extracellular matrix components. It has an inner layer called the lamina rara interna, an external layer called the lamina rara externa, and a central portion called the lamina densa. It is electrically charged and has a specific structure and permeability that allows the passage of molecules that have a molecular weight less than albumin (68,000 daltons). Larger molecules do not pass through the GBM (“selective permeability of the GBM”).
Glomerular basement membrane (GBM), the semipermeable membrane composed of collagen type IV, laminin, and some other extracellular matrix components. It has an inner layer called the lamina rara interna, an external layer called the lamina rara externa, and a central portion called the lamina densa. It is electrically charged and has a specific structure and permeability that allows the passage of molecules that have a molecular weight less than albumin (68,000 daltons). Larger molecules do not pass through the GBM (“selective permeability of the GBM”).
 Visceral epithelial cells (podocytes), which form the foot processes that are tightly linked to the external basement membrane. These cells are in continuity with parietal epithelial cells lining the inside of Bowman’s capsule, thus delimiting them from the glomerular urinary space. The glomerular urinary space freely communicates with the lumen of the proximal tubules.
Visceral epithelial cells (podocytes), which form the foot processes that are tightly linked to the external basement membrane. These cells are in continuity with parietal epithelial cells lining the inside of Bowman’s capsule, thus delimiting them from the glomerular urinary space. The glomerular urinary space freely communicates with the lumen of the proximal tubules.
Renal vessels have several functionally distinct segments.
 Renal arteries. These arteries originate from the abdominal aorta. On entry into the kidneys they branch and give rise to interlobal arteries, which give rise to arcuate arteries running along the corticomedullary junction. Interlobular arteries enter the cortex in the direction of the renal capsule, in their course giving rise to lateral branches called afferent arterioles.
Renal arteries. These arteries originate from the abdominal aorta. On entry into the kidneys they branch and give rise to interlobal arteries, which give rise to arcuate arteries running along the corticomedullary junction. Interlobular arteries enter the cortex in the direction of the renal capsule, in their course giving rise to lateral branches called afferent arterioles.
 Glomerular arterioles. Afferent arterioles enter the glomeruli, branching into the glomerular capillaries, which become confluent on the other end so that the blood that remains following filtration leaves the glomeruli through the efferent arterioles. Efferent arterioles leaving the cortical glomeruli form specialized capillaries.
Glomerular arterioles. Afferent arterioles enter the glomeruli, branching into the glomerular capillaries, which become confluent on the other end so that the blood that remains following filtration leaves the glomeruli through the efferent arterioles. Efferent arterioles leaving the cortical glomeruli form specialized capillaries.
 Peritubular capillary network. This network of capillaries forms from efferent arterioles exiting from high cortical glomeruli. They drain into small renal veins. The arterioles exiting the juxtamedullary glomeruli form the vasa recta, fenestrated capillaries which loop through the medulla before draining into the renal veins.
Peritubular capillary network. This network of capillaries forms from efferent arterioles exiting from high cortical glomeruli. They drain into small renal veins. The arterioles exiting the juxtamedullary glomeruli form the vasa recta, fenestrated capillaries which loop through the medulla before draining into the renal veins.
 Renal veins. Renal veins do not differ significantly from veins in other organs. They drain into the vena cava. Obstruction of the renal veins (e.g., renal vein thrombosis) may adversely affect the function of nephrons.
Renal veins. Renal veins do not differ significantly from veins in other organs. They drain into the vena cava. Obstruction of the renal veins (e.g., renal vein thrombosis) may adversely affect the function of nephrons.
Note that the kidneys have two distinct capillary networks arranged in sequence: a high-pressure one, forming glomerular capillaries and favoring filtration of blood into the urine, and a low-pressure one, composed of the peritubular network and vasa recta, favoring the reabsorption of fluids back into the circulation.
The kidneys contain hormone-secreting cells and cells that activate vitamins.
 Cells of the juxtaglomerular (JG) apparatus. These include the specialized sensor cells of the distal tubule (called the macula densa) and granular renin-secreting JG cells in the outer wall of the afferent arteriole.
Cells of the juxtaglomerular (JG) apparatus. These include the specialized sensor cells of the distal tubule (called the macula densa) and granular renin-secreting JG cells in the outer wall of the afferent arteriole.
 Erythropoietin-secreting cells. These cortical interstitial cells respond to hypoxia by secreting erythropoietin, which stimulates the production and maturation of red blood cells in the bone marrow.
Erythropoietin-secreting cells. These cortical interstitial cells respond to hypoxia by secreting erythropoietin, which stimulates the production and maturation of red blood cells in the bone marrow.
 Vitamin D-activating cells. Vitamin D, which is first hydroxylated in the liver into 25(OH)D3 and released into the blood, is filtered in the glomeruli and reabsorbed by the proximal renal tubules, which hydroxylate it into the active form of vitamin, 1,25(OH)2D3, and release it back into the blood.
Vitamin D-activating cells. Vitamin D, which is first hydroxylated in the liver into 25(OH)D3 and released into the blood, is filtered in the glomeruli and reabsorbed by the proximal renal tubules, which hydroxylate it into the active form of vitamin, 1,25(OH)2D3, and release it back into the blood.
 Angiotensin II-forming cells. Circulating angiotensin I, formed from hepatic angiotensinogen through the action of renin, is taken up by the endothelial cells in renal vessels and transformed into angiotensin II. Angiotensin II is a very potent vasoconstrictor and is important for the autoregulation of renal blood flow. Prostaglandins produced in the kidneys cause vasodilatation and counteract the action of angiotensin II.
Angiotensin II-forming cells. Circulating angiotensin I, formed from hepatic angiotensinogen through the action of renin, is taken up by the endothelial cells in renal vessels and transformed into angiotensin II. Angiotensin II is a very potent vasoconstrictor and is important for the autoregulation of renal blood flow. Prostaglandins produced in the kidneys cause vasodilatation and counteract the action of angiotensin II.
NORMAL PHYSIOLOGY
The principal functions of the kidneys are as follows:
 Formation and excretion of urine
Formation and excretion of urine
 Excretion of waste products, drugs, and toxins
Excretion of waste products, drugs, and toxins
 Regulation of body water and mineral content of the body
Regulation of body water and mineral content of the body
 Maintenance of the acid–base balance
Maintenance of the acid–base balance
 Regulation of blood pressure through the secretion of renin
Regulation of blood pressure through the secretion of renin
 Regulation of hematopoiesis through the secretion of erythropoietin
Regulation of hematopoiesis through the secretion of erythropoietin
The formation of urine depends on proper blood flow and the coordinated function of all parts of the nephron.
The kidneys receive 20% to 30% of the total cardiac output. In practical terms that means that in the average adult male renal plasma flow (RPF) is 600 mL/min. Renal blood flow is autoregulated by the humoral factors, such as angiotensin II, which leads to vasoconstriction, and by prostaglandins, which cause dilatation. At a constant plasma inflow into the glomeruli, the glomerular filtration rate (GFR) also remains constant. Normally, the ratio of RPF to GFR is 5:1, and the GFR is thus 120 mL/min, or approximately 170 L/day. The factors that could reduce the GFR are listed in Table 12-1.
Table 12-1 Causes of Reduced Glomerular Filtration Rate
| PATHOGENETIC MECHANISM | DISEASE |
| Decreased renal blood flow | Heart failure |
| Hypotension–hypoperfusion of kidneys | Massive blood loss |
| Constriction of afferent arterioles | |
| Glomerular inflammation | Acute glomerulonephritis |
| Endothelial cell swelling | Eclampsia |
| Glomerular thrombi | DIC, TTP, ITP |
| Increased urinary back pressure | Urinary obstruction, hydronephrosis |
| Decreased glomerular capillary bed | Chronic glomerulonephritis |
↑, increase; DIC, disseminated intravascular coagulation; ITP, idiopathic thrombocytopenic purpura; TTP, thrombotic thrombocytopenic purpura.
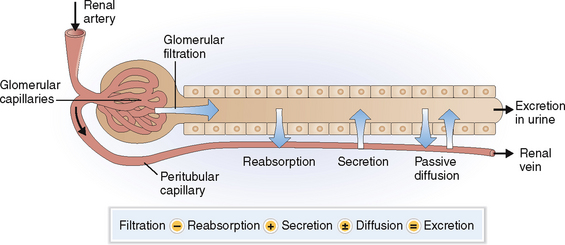
The excretion of urine is a stepwise process that includes several site-specific physiologic events aimed at preserving the essential balance of minerals and organic compounds and eliminating the waste products (Fig. 12-3):
Filtration. The plasma filtered in the glomeruli forms the glomerular filtrate, which enters into the lumen of the proximal tubules. The red blood cells (RBCs) and the white blood cells (WBCs) cannot pass the endothelial cell barrier and are thus not found in the glomerular filtrate. The semipermeable GBM allows only the passage of proteins that have molecular weights lower than albumin (68,000 daltons). Almost all proteins reaching the proximal tubule are reabsorbed, and the final urine contains thus less than 150 mg of protein per 24 hours.
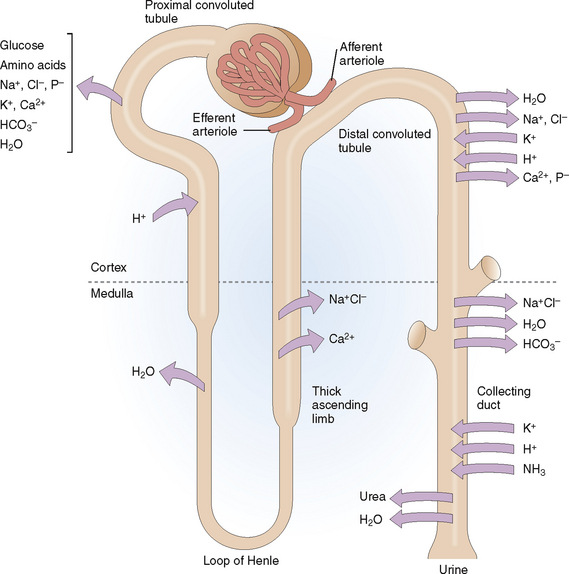
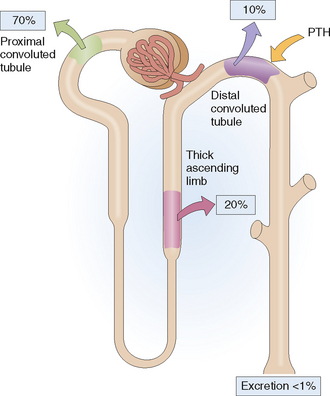
Reabsorption. Different minerals and metabolites are handled by different parts of the nephrons (Fig. 12-4). The proximal tubule is the most active site and thus reabsorbs almost all the amino acids, glucose, and bicarbonate and 70% of the sodium, potassium, calcium, and phosphate. Additional reabsorption of calcium in the distal tubule is regulated by parathyroid hormone (PTH) and the reabsorption of sodium by aldosterone. Reabsorption of sodium phosphate in the proximal tubule may be inhibited by PTH. The reabsorption of water in the collecting duct occurs under the control of antidiuretic hormone (ADH), also known as vasopressin.
Passive diffusion. Water passively follows the movement of sodium and chloride in most parts of the nephron or moves toward areas of high osmolality. Diffusion of water out of the tubules can occur anywhere except in the ascending loop of Henle, which is impermeable to water. Urea, the concentration of which increases progressively as the filtrate passes along the collecting duct, also diffuses from the distal parts of the collecting duct into the interstitial fluid of the medulla, which has a lower concentration of urea than the lumen of the collecting ducts.
The kidneys conserve or excrete water according to the needs of the body.
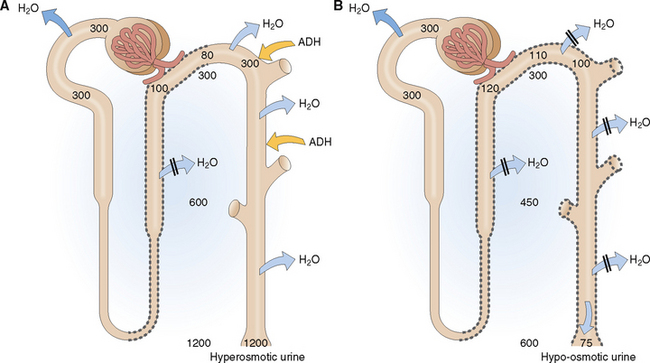
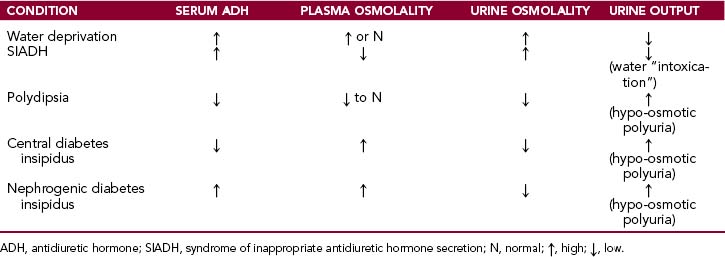
Urine produced in the presence of high ADH concentration in the blood is hyperosmotic (Fig. 12-5). Remember that the primary glomerular filtrate is isosmotic with plasma (285–295 mOsm/kg). The water in the glomerular filtrate moves out of the proximal tubule in parallel with the minerals that are reabsorbed, so that the osmolality of the intratubular fluid does not change until the fluid enters the thick ascending limb of the loop of Henle and the early distal tubules. These parts of the nephron are impermeable to water, and thus the reabsorption of Na+Cl− is not followed by water. Accordingly the tubular fluid entering the late distal tubule is diluted. This hyposmolar tubular fluid diffuses through the wall of the late distal tubule, because it is made water-permeable by ADH. Antidiuretic hormone-mediated water diffusion continues to occur in the collecting duct as well, leading to a concentration of the tubular contents all the way to 1200 mOsm/kg.
Hypo-osmotic urine is produced in the presence of low ADH levels in blood. Antidiuretic hormone diminishes somewhat the tubular fluid in the thick ascending limb of the loop of Henle and the early distal tubule, but the most prominent effects of ADH deficiency are seen in the late distal tubule and the collecting duct. Without ADH these parts of the nephron become impermeable to water, and water is excreted in large amounts, thereby diluting the urine.
The salient features of the main clinical conditions related to ADH water conservation or loss are listed in Table 12-2.
The kidneys can modify the excretion of sodium to maintain a constant serum concentration of sodium.
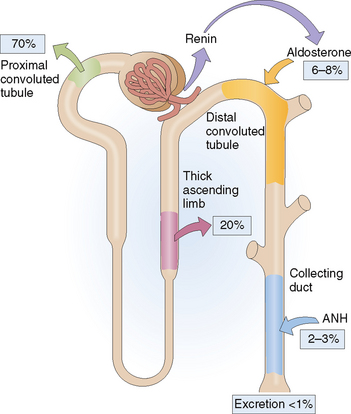
Sodium is filtered in the glomeruli in large amounts, but more than 99% of that Na+ is reabsorbed in the nephron (Fig. 12-6). Actually, even the small amount of Na+ that is excreted in the urine can be conserved to a great extent if need be, as is the case when extrarenal Na+ loss increases in the gastrointestinal tract or through sweating.
The later part of the distal tubule and the collecting ducts reabsorb 6% to 8% of filtered Na+. Nevertheless, this part of the nephron is very important in regulating Na+ excretion, because these cells function under the control of aldosterone. Aldosterone increases Na+ reabsorption and increases K+ secretion. Atrial natriuretic hormone (ANH) promotes renal Na+ excretion by inhibiting Na+ reabsorption in the inner medullary collecting duct. Atrial natriuretic hormone acts on other parts of the kidney, such as by dilating the afferent arterioles increasing the GFR, by increasing the medullary blood flow, and by decreasing the release of renin. Atrial natriuretic hormone also inhibits the secretion of aldosterone and the release of ADH.
Potassium excretion in the urine is predominantly regulated by aldosterone, the requirements for sodium excretion, and acid–base status.
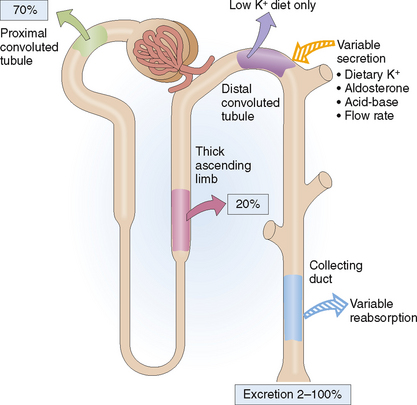
Both K+ and Na+ are filtered in the glomeruli, and the glomerular filtrate contains the same concentration of these electrolytes as the plasma. As in plasma the concentration of K+ is much lower. Nevertheless, like sodium, the K+ is mostly (approximately 70%) reabsorbed in the proximal tubules (Fig. 12-7). Like Na+ it is also reabsorbed in the ascending loop of Henle and in the distal nephron. Aldosterone stimulates the distal tubule and the collecting ducts to secrete K+, which is diametrically opposite from the effects of aldosterone on Na+. This typically occurs when the blood concentration of potassium is increased due to high dietary intake of K+ or in acidosis. Under normal conditions urine contains only 2% of the K+ that was initially filtered in the glomeruli, but in hyperkalemia its concentration in urine may increase 50 times.
Calcium and phosphorus concentrations in the serum critically depend on renal excretion of these minerals.
Calcium circulates in the blood in a free form and bound to albumin. Free calcium (Ca2+) is filtered in the glomeruli and most of it is reabsorbed in the proximal tubule (Fig. 12-8). The remaining Ca2+ reaches the distal parts of the nephron, which reabsorb most of it, allowing only 1% of the filtered Ca2+ to be excreted. The excretion of Ca2+ is under the influence of PTH, which promotes Ca2+ reabsorption in the distal tubule (“hypocalciuric effect of PTH”). Calciuria may occur in hypercalcemia, when more Ca2+ is filtered in the glomeruli. Natriuresis and acidosis also increase Ca2+ excretion in the urine.
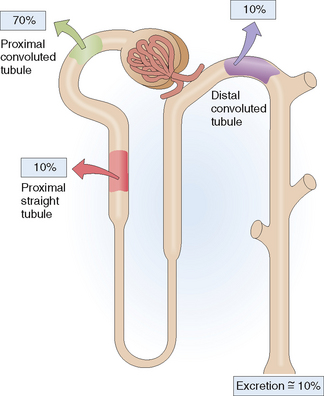
Phosphorus circulates in the blood as phosphate or bound to proteins. Phosphorus, mostly in form of phosphates, is filtered in the glomeruli, and up to 90% of it is reabsorbed in the nephron (Fig. 12-9). Most of the reabsorption (up to 70%) occurs linked to Na+ (“sodium–phosphate cotransport”) in the proximal convoluted tubule and to a lesser extent (up to 10%) in the proximal straight tubule. Approximately 10% of phosphorus is absorbed in the distal tubule. Parathyroid hormone inhibits the cotransport of sodium and phosphate, promoting the excretion of phosphorus (“phosphaturic effect of PTH”).
The kidneys are important for the maintenance of acid–base balance.
 Excretion of fixed acids. These acids are produced from incomplete oxidation of proteins, lipids, and carbohydrates.
Excretion of fixed acids. These acids are produced from incomplete oxidation of proteins, lipids, and carbohydrates.
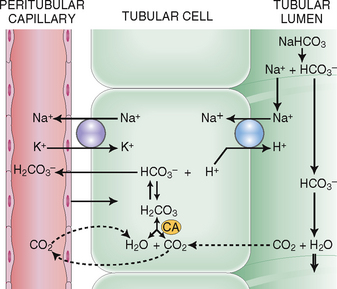
 Reabsorption of bicarbonate and excretion of hydrogen. Eighty percent to 90% of bicarbonate (HCO3−) filtered in the glomeruli is reclaimed in the proximal tubules. Active secretion of H+ in the distal tubules serves to exchange H+ for HCO3− and thus save the remaining 10% to 20% of filtered HCO3− (Fig. 12-10). Sodium is used in this interchange and is linked to the transport of K+. Hydrogen reacts in the tubular fluid with HCO3−, thus forming H2CO3, which dissociates into CO2 and H2O. Free CO2 diffuses back into the cytoplasm of the tubular cells and under the action of carbonic anhydrase again forms HCO3−, which is returned into the blood.
Reabsorption of bicarbonate and excretion of hydrogen. Eighty percent to 90% of bicarbonate (HCO3−) filtered in the glomeruli is reclaimed in the proximal tubules. Active secretion of H+ in the distal tubules serves to exchange H+ for HCO3− and thus save the remaining 10% to 20% of filtered HCO3− (Fig. 12-10). Sodium is used in this interchange and is linked to the transport of K+. Hydrogen reacts in the tubular fluid with HCO3−, thus forming H2CO3, which dissociates into CO2 and H2O. Free CO2 diffuses back into the cytoplasm of the tubular cells and under the action of carbonic anhydrase again forms HCO3−, which is returned into the blood.
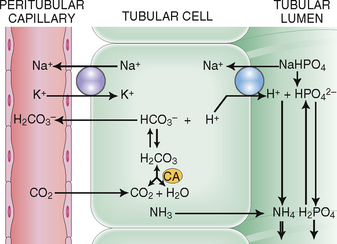
 Excretion of ammonia and phosphate. Hydrogen ions excreted into the tubular fluid must be buffered, which is achieved by the secretion of ammonia and filtered phosphates (Fig. 12-11).
Excretion of ammonia and phosphate. Hydrogen ions excreted into the tubular fluid must be buffered, which is achieved by the secretion of ammonia and filtered phosphates (Fig. 12-11).
The kidneys excrete metabolic waste products, drugs, and toxins.
Urea. It is filtered in the glomeruli, and approximately one half of it is reabsorbed in the proximal tubule. The ascending loops of Henle secrete urea into the lumen, the distal tubules resorb 30%, and then finally the inner medullary collecting duct resorbs large amounts of the remaining urea. Hence only 15% of the filtered urea is excreted. In conditions that increase urine production urea excretion may increase up to 60% of the filtered urea concentration.
The kidneys have endocrine functions.
 Renin. This blood-regulating hormone is produced by the JG apparatus cells. It acts on angiotensinogen, transforming it into angiotensin I. Angiotensin I is transformed into angiotensin II through the action of angiotensin-converting enzyme. Angiotensin II acts on the adrenal cortex to produce aldosterone. At the same time angiotensin II is a potent vasoconstrictor and has several other effects on the kidneys.
Renin. This blood-regulating hormone is produced by the JG apparatus cells. It acts on angiotensinogen, transforming it into angiotensin I. Angiotensin I is transformed into angiotensin II through the action of angiotensin-converting enzyme. Angiotensin II acts on the adrenal cortex to produce aldosterone. At the same time angiotensin II is a potent vasoconstrictor and has several other effects on the kidneys.
 Erythropoietin. This hormone acts a growth factor for erythroid precursors of the bone marrow and also promotes differentiation and maturation of these cells.
Erythropoietin. This hormone acts a growth factor for erythroid precursors of the bone marrow and also promotes differentiation and maturation of these cells.
 1,25(OH)2D3 vitamin. This active form of vitamin D is produced in the kidneys and released into the circulation, where, in tandem with PTH, it regulates the homeostasis, absorption, and deposition of calcium.
1,25(OH)2D3 vitamin. This active form of vitamin D is produced in the kidneys and released into the circulation, where, in tandem with PTH, it regulates the homeostasis, absorption, and deposition of calcium.
Clinical and Laboratory Evaluation of Renal Disease
FAMILY AND PERSONAL HISTORY
Family and personal history can point to some important risk factors that play a role in the pathogenesis of renal diseases. A few examples of such links and associations are given in Table 12-3.
Table 12-3 Risk Factors for Renal Diseases
| TYPE OF RISK FACTOR | SPECIFIC DISEASES—RISK FACTOR ASSOCIATIONS |
| Hereditary factors | |
| Social factors/infections | |
| Other systemic diseases or diseases of other organ systems | |
| Environment | Hot climate: Higher incidence of renal stones in the “stone belt” states in the southern United States |
| Surgical procedures | |
| Drugs | |
| Pregnancy |
HIV, human immunodeficiency virus; IV, intravenous.
PHYSICAL EXAMINATION AND HISTORY OF PRESENT DISEASE
Frequency of urination and the volume of urine produced vary under normal conditions and if increased may be a sign of disease.
The frequency of urination and total urine output depend on several factors, including
 Fluid intake. Fluid intake depends on individual drinking habits and may be increased considerably under stress or in psychogenic polydipsia, a condition characterized by compulsive drinking. Bear in mind that alcoholic beverages contain not only alcohol but also water, and that a typical heavy beer drinker may consume huge amounts of extra water a day.
Fluid intake. Fluid intake depends on individual drinking habits and may be increased considerably under stress or in psychogenic polydipsia, a condition characterized by compulsive drinking. Bear in mind that alcoholic beverages contain not only alcohol but also water, and that a typical heavy beer drinker may consume huge amounts of extra water a day.
 Extrarenal fluid loss. Fluid may be lost due to insensible evaporation (e.g., in airplanes), increased sweating (e.g., hot weather), and gastrointestinal loss (e.g., diarrhea or vomiting).
Extrarenal fluid loss. Fluid may be lost due to insensible evaporation (e.g., in airplanes), increased sweating (e.g., hot weather), and gastrointestinal loss (e.g., diarrhea or vomiting).
 Renal function. Loss of renal function may reduce or increase the volume of urine. Polyuria is associated with increased frequency of urination.
Renal function. Loss of renal function may reduce or increase the volume of urine. Polyuria is associated with increased frequency of urination.
 Capacity of the urinary bladder. The bladder is somewhat smaller in women than in men, but even so its capacity varies from one person to another. Chronic infections and hypertrophy due to obstruction of the bladder neck reduce the capacity of the bladder.
Capacity of the urinary bladder. The bladder is somewhat smaller in women than in men, but even so its capacity varies from one person to another. Chronic infections and hypertrophy due to obstruction of the bladder neck reduce the capacity of the bladder.
 Drugs. Diuretics and many other drugs affect the renal handling of salts and water and thus influence urine production.
Drugs. Diuretics and many other drugs affect the renal handling of salts and water and thus influence urine production.
 Local conditions modifying the micturition reflex. The micturition reflex is initiated by stimuli acting on sensory nerves in the posterior urethra and bladder neck. Irritation of these nerve endings by inflammation, urinary stones, or prostatic hyperplasia is associated with increased frequency.
Local conditions modifying the micturition reflex. The micturition reflex is initiated by stimuli acting on sensory nerves in the posterior urethra and bladder neck. Irritation of these nerve endings by inflammation, urinary stones, or prostatic hyperplasia is associated with increased frequency.
 Frequent urination (pollakisuria) can be divided clinically into two major groups: urination with polyuria and urination without polyuria. The causes of polyuria are listed in Table 12-4.
Frequent urination (pollakisuria) can be divided clinically into two major groups: urination with polyuria and urination without polyuria. The causes of polyuria are listed in Table 12-4.
Table 12-4 Causes of Frequent Urination
| FREQUENT URINATION WITHOUT POLYURIA | FREQUENT URINATION WITH POLYURIA |
Get Clinical Tree app for offline access 
|