CELLS OF THE IMMUNE RESPONSE SYSTEM
The immune response is under the control of specialized cells, the
white blood cells. White blood cells protect the body from infection and cancer and assist in healing. White blood cells include the neutrophils, eosinophils, basophils, monocytes, and macrophages, and the B and T lymphocytes. Platelets are fragments of cells that also play a role in healing. All white blood cells and
platelets derive from a basic stem (originator) cell, called the pluripotent stem cell, in the bone marrow. From this cell, succeeding generations of stem cells differentiate and commit to producing one type of cell. Normal values for selected blood components are included in
Table 4-1. Blood cells and their differentiation are discussed in
Chapter 12.
Neutrophils, Eosinophils, and Basophils
Neutrophils, eosinophils, and basophils are called granulocytes because of the cytoplasmic granules that dominate their appearance under the microscope. The granulocytes remain in the bone marrow or circulation until they are drawn to an area of infection, inflammation, or trauma by substances released from damaged tissues, by microorganisms, or by the B or T lymphocytes. The cytoplasmic granules contain enzymes that break down and destroy microorganisms and digest cellular debris. Once granulocytes complete their function, they die. In a serious infection, granulocytes may only survive a few hours.
The neutrophils are the first white blood cells to arrive at an area of injury or infection and are key players in the processes of inflammation. Neutrophils arriving on the scene begin phagocytizing the cells and debris immediately. They also release chemicals that damage microorganisms and attract other white blood cells to the area, in a process called chemotaxis. Neutrophils initiate the inflammatory responses of vasodilation and increased capillary permeability. Clinically, neutrophils are often referred to as polymorphonuclear cells (PMNs) or segmented neutrophils (“segs”) because of the segmented appearance of their multilobed nuclei. Eosinophils have several functions. First, they are involved in the allergic response (described later). Second, they are important in the defense against parasitic (helminthic) infections. They also protect the host by reducing the inflammatory response by destroying histamine. The eosinophils phagocytize cell debris, although to a lesser degree than do neutrophils. The level of eosinophils may increase during an allergic response or in response to helminthic infection.
Basophils circulate in the bloodstream and, when activated by injury or infection, release histamine, bradykinin, and serotonin. These substances increase capillary permeability and blood flow to the area, bringing to the area other mediators required to eliminate infection and promote healing. Basophils secrete the natural anticlotting substance heparin, which ensures that clotting and coagulation pathways do not continue unchecked. Basophils are also involved in producing allergic responses. They are similar in function to important initiators of tissue inflammation, the mast cells, but unlike mast cells, basophils circulate in the blood.
Monocytes and Macrophages
Monocytes circulate in the blood and enter injured tissue across capillary membranes that become permeable as part of the inflammatory reaction (described later). They arrive at the site of injury hours to days after neutrophils. Monocytes are not phagocytic, but after several hours in the tissue area, they mature into macrophages. Macrophages are large cells capable of withstanding an acidic environment and ingesting large quantities of cell debris and bacteria. Macrophages can phagocytize lysed red blood cells and other white blood cells. Some macrophage cells colonize tissues such as skin, lymph nodes, and lungs for months or years. These cells are readily available to scavenge microorganisms that may enter the body through those routes. The monocyte-macrophage cell system is called the reticuloendothelial system.
Lymphocytes
Lymphocytes include the B and T lymphocytes and a type of cell called the natural killer (NK) cell. Lymphocytes are produced in the bone marrow and mature there or in other lymphoid tissues.
B lymphocytes (B cells) mature in the bone marrow. After maturation, a B cell circulates in the blood in an inactive state and becomes active only after exposure to a specific molecule, usually a protein or large carbohydrate of foreign origin, to which it has been genetically programmed during fetal development to respond. When activated, the B cell becomes a plasma cell, a specialized cell that mounts an immune response against the molecule that activated it. B lymphocytes comprise the humoral immune system, meaning that they circulate in the blood (the humor).
T lymphocytes comprise the cellular immune system. T-cell maturation occurs during passage through the thymus gland. Like a B cell, the mature T cell stays inactive until it encounters the specific molecule to which it has been programmed during development to respond; once it does so, it becomes activated and may directly attack and destroy the cell expressing that molecule. The T cell may also release chemicals that alert B cells to the presence of the invader, thereby initiating a humoral response. T cells can stimulate or in some circumstances inhibit the inflammatory responses via the release of pro- or
anti-inflammatory peptides known as
cytokines. T cells are important for recognizing and destroying parasites and viruses that hide intracellularly, where the B cells are unable to encounter them.
NK cells are produced in the bone marrow and provide innate defense for the body. The NK cells react to foreign molecules, but do not demonstrate specificity, that is, they may respond to more than one foreign molecule. They recognize abnormal cells by one of two mechanisms: killer-activating receptors or killer-inhibiting receptors. The natural history of NK cell origin and maturation is unclear.
Platelets
Platelets are not cells, but cytoplasmic fragments that develop from specialized cells in the bone marrow called megakaryocytes. Like white blood cells, platelets are drawn to an area of inflammation. Once the platelets arrive at the site of injury, they adhere to the vessel wall, forming aggregates or plugs. Adhering to the vessel wall activates the platelets, causing them to release several biochemical mediators, including serotonin and histamine, which temporarily decrease blood flow and bleeding. This vasoconstriction is short lived, however, and soon blood flow increases to deliver other white blood cells to the area. If the injury is small, the platelet plug is usually sufficient to allow for healing. Platelets circulate in the blood for about 10 days before they become nonfunctioning and are phagocytized by neutrophils and monocytes. If a person has too few platelets, he or she is at increased risk of developing multiple small hemorrhages under the skin and throughout the body.
THE SPECIFIC AND INNATE IMMUNE RESPONSES
The immune system includes both specific and innate responses. Specific immune responses are those that involve activation of the B and T lymphocytes. B and T lymphocytes are capable of responding with specificity and precision to virtually any foreign molecule an individual may encounter in a lifetime. Once the original response is made, the B or T cell retains a memory of it. If a second encounter with that molecule occurs, the B- or T-cell response will be faster and more effective than before.
The innate immune response, in contrast, includes the inflammatory responses to infection or injury and the white blood cells that participate in those responses: the neutrophils, basophils, eosinophils, and monocytes and macrophages. Species resistance, mechanical barriers, chemical barriers, secretions, inflammation, interferon, and complement are all part of the innate immune system. The inflammatory response is stimulated after tissue injury or infection, with the goal of delivering white blood cells and platelets to the tissues to limit damage and promote healing. The inflammatory reactions are not characterized by specificity or memory, but they are fast and effective.
ANTIGENS
An antigen is any molecule that can stimulate a specific immune response against itself or the cell that carries it. Billions of B and T lymphocytes are produced during fetal development with the potential to bind to at least 100 million distinct antigens. Antigens that can bind to a T or B lymphocyte include those present on the cell wall of bacteria or mycoplasmas, the coat of a virus, or on certain pollens, dusts, or foods. Every cell of a person has surface proteins that would be recognized as foreign antigens by B or T lymphocytes from another person. If an antigen causes either the B or T lymphocyte to become activated and to multiply or differentiate further, it is an immunogenic antigen.
B-LYMPHOCYTE RESPONSE TO AN ANTIGEN
When a B lymphocyte encounters its specific antigen, it binds to it in a “lock and key” fashion, causing the B cell to differentiate into a plasma cell. The plasma cell in turn begins to secrete millions of molecules of antibodies made specifically against that antigen. Once produced, the antibodies, also called immunoglobulins, circulate throughout the bloodstream seeking to eliminate the antigen that stimulated their production. Antibody-mediated responses are important for defense against bacteria and circulating viruses and against toxins released from bacteria.
Immunoglobulins/Antibodies
There are five specific immunoglobulins produced in response to an antigen: IgG, IgM, IgA, IgE, and IgD. A description of each is included in
Table 4-2.
Antibody Structure
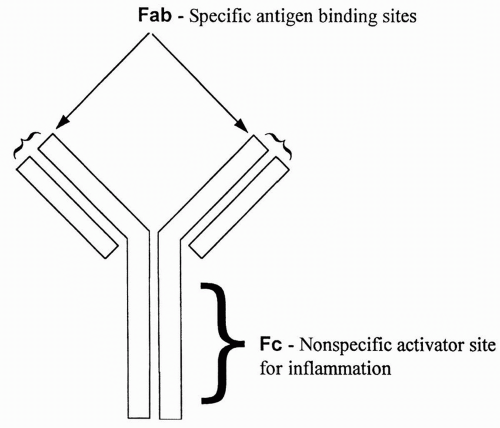
All antibodies are similar in appearance. They consist of two long heavy chains called the Fc portion, and two small heads called the Fab portion. The Fc portion is identical for all antibodies of a single class (e.g., IgG or IgM). The Fab portion is specific for each antibody and contains the specific binding site for an antigen. Binding of antigen to the Fab portion of the antibody activates the Fc portion, leading to destruction of the microorganism or other antigen-bearing cell. An antibody is shown in
Figure 4-1.
Antibody Destruction of a Microorganism
Antibodies cause the destruction of bound antigen by a variety of mechanisms. Usually, the antibody does not kill the cell, but instead coordinates the attack by turning on NK cells, activating complement, and enhancing phagocytosis. Under some circumstances, an antibody may directly inactivate an antigen.
NK cell activation occurs when binding of the antigen to the Fab (specific) portion of the antibody allows an NK cell to establish connections with the
Fc (nonspecific) portion, thus linking up the NK cell with the antigen (
Fig. 4-2). The NK cell then releases toxic chemicals that directly kill the antigen target.
Complement activation will be described in more detail shortly. Complement is a series of molecules that, when activated, leads to the initiation of an inflammatory response and the killing of the antigen-bearing cell. Like NK cell activation, binding of the antigen to the Fab portion of the antibody allows the first molecule in the complement chain (C1) to bind nonspecifically to the Fc portion. Such binding hooks up the antigen-bearing cell with the complement, ultimately leading to the destruction of the antigen-bearing cell.
Phagocytic stimulation occurs similarly; when the antigen binds to the Fab portion of the antibody, this allows a phagocytic cell (usually a macrophage or neutrophil) to bind to the nonspecific Fc portion, stimulating phagocytosis of the linked antigen and the cell that bears it.
Direct effects of an antibody may occur if, for example, an antibody binds to a virus at the same site that the virus uses to bind to and enter a susceptible cell. This would inactivate the virus. Similarly, the antibody may bind to a bacterial toxin at the same site that the toxin would use to interact with susceptible cells. This would eliminate the effect of the toxin.
Opsonization
Binding of an antibody to an antigen on a bacterium causes opsonization, a change in the bacterial cell wall that renders otherwise impenetrable bacteria susceptible to phagocytosis. The complement also serves as an opsonin (an agent that can cause opsonization).
The Role of the T Cell in B-Cell Response to an Antigen
To mount an antibody attack against a microorganism, T-cell support is almost always required. As described below, cytokines released by activated T lymphocytes trigger B-cell proliferation and differentiation into antibody-secreting plasma cells.
Memory Cells
Some B lymphocytes do not become antibody-secreting plasma cells after antigenic stimulation, but rather become memory cells. Memory cells circulate indefinitely in the blood and become active immediately upon repeated exposure to the antigen.
The first time a B lymphocyte is exposed to its antigen (the primary exposure), production of antibodies against the antigen can take 2 weeks to more than 1 year, although normally antibodies to an antigen are detectable in the blood within 3 to 6 months. Because of memory cells, the next time the antigen is encountered, the antibody response occurs almost immediately (see
Fig. 4-3).
T-LYMPHOCYTE RESPONSE TO AN ANTIGEN
When a T lymphocyte binds to an immunogenic antigen, it is stimulated to mature and reproduce. This reproduction results in up to four subtypes of
T cells capable of acting in response to the antigen: cytotoxic T cells, helper T cells, regulatory T cells, and memory T cells. The T-cell response to antigen is called a cell-mediated response, because the T cells respond directly; they do not need to become plasma cells and secrete antibody to destroy the antigen.
Cytotoxic T cells directly destroy the antigen by releasing toxic chemicals. These chemicals punch holes in the membranes of the cells carrying the foreign antigen. Cytotoxic T cells are also called CD8 cells because of a specific protein present on their plasma membrane.
Helper T cells secrete peptides, called cytokines, which act as cell messengers to coordinate the response of cytotoxic T cells and B cells. There are two general categories of helper T (Th) cells: Th1 and Th2 cells. Th1 cells release cytokines that are proinflammatory, in that they draw neutrophils and monocytes to the area of injury or infection and stimulate macrophage phagocytosis. Th1 cytokines increase the production of prostaglandins, leading to increased blood flow and interstitial edema, and induce systemic symptoms of inflammation, including fever. Th1 cytokines favor the production of cytotoxic T cells and induce cell-mediated immune responses. Th2 cells generally secrete anti-inflammatory cytokines, which put the brakes on potentially dangerous inflammatory reactions. Th2 cells favor activation of humoral (B-cell driven) responses. Normally, Th1 and Th2 immune responses are in balance with each other.
Regulatory T cells act to suppress the host’s immune response, a function that under some circumstances may increase the risk of infection, but under other circumstances may serve to protect the host against an overzealous immune system. Although their mechanism of action is still under investigation, regulatory T cells appear to suppress immune function via direct contact with B cells or other T cells, and/or by releasing anti-inflammatory cytokines. A deficiency in regulatory T cells has been suggested to play a role in the development of autoimmune disease, while overactive regulatory T cells may protect tumor cells from immune attack. Some evidence suggests that certain viruses, including the human immunodeficiency virus (HIV), exploit regulatory T cells’ ability to dampen the body’s antiviral response. Regulatory T cells are characterized by C25 receptors on their cell membranes.
Memory T cells circulate in the bloodstream until the specific antigen that stimulated their production is encountered again. Subsequent responses to that antigen occur rapidly.
RECOGNITION OF SELF VERSUS FOREIGN ANTIGENS
It is essential that the immune system recognizes self-antigens and only initiates attack against nonself or damaged cells. To ensure only appropriate immune
responses, potential antigens are always presented to the immune system, and to the T cells in particular, in combination with self-antigens, called major histocompatibility complex (MHC) proteins. Because these antigens are expressed in such high concentrations on leukocytes, they are often referred to as human leukocyte antigens (HLA).
Self-Antigens
Each individual possesses cell surface antigens that are unique to that individual. These antigens, the MHC proteins or histocompatibility antigens, serve as a sort of cellular fingerprint. There are two groups of MHC proteins: MHC I and MHC II. The MHC I proteins are found on nearly all cells of the body except the red blood cells. The MHC II proteins are found only on the surface of macrophages and B cells. MHC proteins have two functions: (1) they present self-antigens to T cells and (2) they bind foreign antigens and present these to T cells. The MHC I molecules bind and present antigens only to cytotoxic T cells. The MHC II molecules bind and present antigens only to helper T cells (both Th1 and Th2 types).
The MHC Genes
The MHC proteins are inherited as four closely linked loci (groups of genes) on chromosome 6. These genes, called the MHC, are usually inherited together, with one set of loci received from each parent. There are many different possible alleles for each loci, resulting in more than one trillion possible antigen combinations. Therefore, it is virtually impossible for two unrelated individuals to have matching MHC proteins. Identical twins will have the same proteins, and an individual’s siblings and offspring will likely have MHC proteins that are more similar than those of unrelated individuals.
The Role of the MHC Proteins in Controlling Immunity
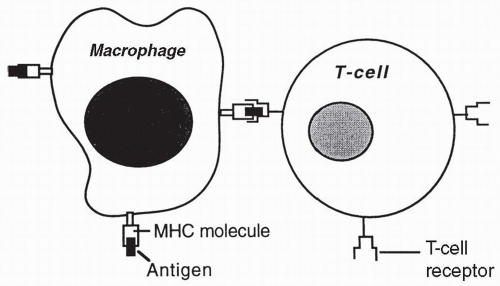
After a foreign or unknown cell has been phagocytized by a macrophage or has become bound to a B cell, antigens from the cell are expressed on the macrophage or B cell adjacent to host MHC II antigens. The foreign antigens and the MHC II antigens are presented together to passing helper T (CD4) cells. Each passing helper T cell compares the foreign or unknown antigen to the host’s MHC II antigens (
Fig. 4-4). If in comparing the unknown antigen to the MHC II antigens, a helper T cell recognizes the antigen as foreign, the helper T cell will secrete cytokines that activate the B cell to become an antibody-secreting plasma cell. If the antigen presented is seen by helper T cells as too similar to the MHC II proteins on the B cell or macrophage, the helper T cells will not become activated, or perhaps may become T regulatory cells, and the antigen will not be attacked.
To activate cytotoxic (CD8) cells, MHC I proteins must be presented in association with the unknown or foreign antigen. All cells express MHC I proteins; therefore, any cell can present foreign antigens to CD8 cells for comparison. Cells infected with a virus make abnormal proteins, as do cancerous cells. These abnormal proteins are recognized as antigens and presented to CD8 cells along with host MHC I proteins. When cytotoxic T cells encounter abnormal proteins compared to the MHC I proteins, they are stimulated to initiate killing of the cells.
Graft Rejection and MHC Proteins
A poor match of MHC proteins is the major cause of graft rejection. Not all cellular antigens need to match; however, the closer the MHC profile match between donor and host, the greater the chance a graft will be accepted. Graft rejection is an example of cell-mediated immunity.
Development of Self-Tolerance
During gestation, hundreds of thousands of T and B cells are formed. Some of these T and B cells fit lock and key with host antigens and are therefore capable of reacting against them. To eliminate the potential of attack against host cells, T cells residing in the thymus and B cells in the bone marrow are exposed during a critical period of embryogenesis to a multitude of host antigens. If, during this time, a B or T cell encounters an antigen to which it matches, the B or T cell is programmed to undergo apoptosis and self-destruct. This leaves behind only cells tolerant to host antigens. This theory of tolerance is called the clonal deletion theory because it explains the elimination of clones of immune cells that react with self-antigens.
A second method also exists to ensure the elimination of cells with the potential to attack host antigens. This mechanism, called clonal inactivation, occurs outside the thymus during fetal development and throughout life. In this scenario, MHC II antigens are presented to helper T cells. If a helper T cell encounters the specific antigen to which it matches among the MHC proteins, the helper cell undergoes apoptosis.
Because helper T cells are essential in activating B cells to become plasma cells, clonal deletion and clonal inactivation of T cells can eliminate humoral and cellular immunity against self-antigens. In these processes, tolerance is recognized as an active process, essential for the survival of the host. Occasionally, tolerance to host cells may be lost, which leads to the development of an immune response against those cells and may cause autoimmune disease.
Exceptions to Clonal Elimination
Some tissues grow during fetal development without exposure to immature T cells. These tissues normally are kept sequestered from the immune system after birth. If they are later exposed to immune cells, however, an attack against them may occur. Cells that are normally kept sequestered from the immune system include certain cells of the testes and eye.
B- AND T-LYMPHOCYTE RESPONSES TO A FOREIGN ANTIGEN
In a B-cell response, B cells bind a foreign antigen. Helper T cells are presented pieces of the antigen, either by the B cell directly or by macrophages that have begun phagocytizing the antigen. If the antigen is different enough from the MHC II proteins expressed by the B cell or macrophage, the helper T cell releases cytokines that activate the B cell, causing it to become an antibody-secreting plasma cell. The antibody will bind the antigen throughout the body and orchestrate its destruction. The T cells also stimulate macrophages to increase phagocytosis of the organism and activate other white blood cells and complement to assist in the defense response.
In a cell-mediated response, cells of any type infected with an intracellular organism or cells that have become cancerous present foreign proteins to cytotoxic T cells along with their own MHC I proteins. This activates the cytotoxic cells to destroy the cells carrying the foreign protein.
RED BLOOD CELL ANTIGENS
There are at least 80 different antigens present on the red blood cells. The most important of these are the ABO antigens and the Rh antigens. Blood types are referred to as ABO and Rh.
ABO Antigens
The ABO blood group consists of A and B antigens. An individual receives from each parent an A antigen gene and a B antigen gene, or neither (called the O antigen). The A and B antigen genes are each dominant over the O antigen gene, but are codominant with each other. Thus, an individual who receives an A antigen gene from one parent and an A or an O from the other (AA or AO) will have type A blood. An individual who receives a B antigen gene from one parent and a B or an O from the other (BB or BO) will have type B blood. An individual who receives an A antigen gene from one parent and a B antigen gene from the other will have AB blood. Type O blood is possible only if an individual receives neither the A nor B antigen gene from either parent (OO).
Rh Antigens
The Rh antigens are a complex group of antigens present on the red blood cell. If a particular type of Rh antigen is present on the red blood cells, an individual is considered to be Rh positive. If this antigen is not present, an individual is considered Rh negative. Each individual receives one Rh gene from each parent. The Rh-positive gene dominates, such that an individual with one Rh-positive gene and one Rh-negative gene is Rh positive. Rh antigens, when mismatched between mother and fetus, can be responsible for a severe reaction in the fetus. This reaction is characterized by red blood cell lysis and anemia (hemolytic disease of the newborn and erythroblastosis fetalis), which occurs when an Rh-negative mother produces antibodies against the red cells of an Rh-positive fetus. Antibodies may cross the placenta and cause destruction of fetal red blood cells before or during birth.
Immune Reactions against Mismatched Blood
An individual with type A blood given a transfusion of type B blood may develop a severe immune reaction against the B blood, called a transfusion reaction. In a transfusion reaction, lysis and agglutination of the donated red blood cells occur. Inflammation, blood clotting, and death may result. A similar reaction would occur if an individual with type B blood were to receive a donation of type A blood. An individual with Rh-negative blood who receives an Rh-positive blood transfusion may also have an immunologic reaction, although typically the response is less intense.
Individuals with type A or type B blood can safely receive type O blood because type O blood will not stimulate an antibody reaction. Individuals with O-negative blood are called universal donors because they can supply blood to anyone. Individuals with AB-positive blood are universal recipients because they will not react against any type blood. O-negative is the blood of choice
for an emergency trauma patient who has not been cross-matched (blood type determined). See
Table 4-3 for a summary of blood types and transfusion compatibility.