INTRODUCTION
The role of the pathologist in diagnosing gynecologic disease includes a broad range of conditions from infectious to congenital and from benign to malignant neoplasms in all parts of the female reproductive tract. The following two cases are typical of those seen in the practice of gynecologic pathology.
CASE 17-1
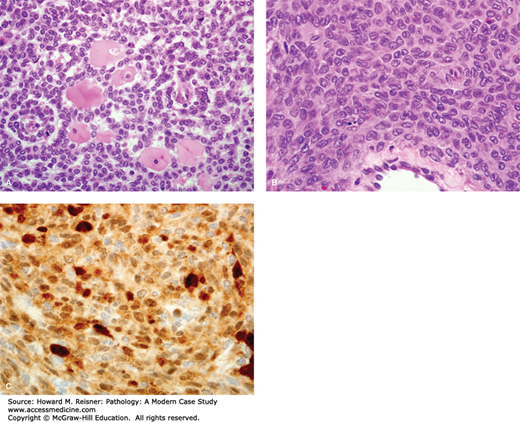
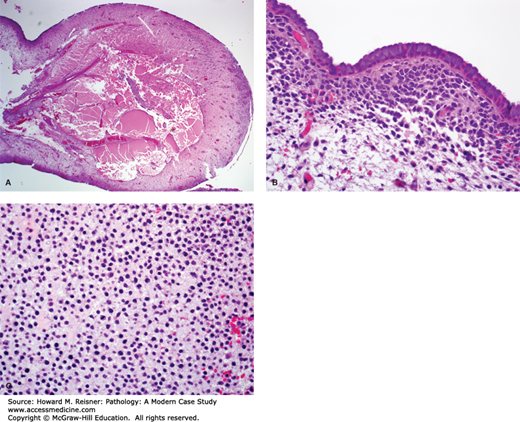
A 57-year-old woman presents to her gynecologist with the complaint of postmenopausal bleeding. During her workup, a CT scan of her abdomen shows a 12-cm right ovarian mass. Clinical lab values drawn prior to removal of the mass include a markedly elevated inhibin level. Photos of the tumor are shown below (Figure 17-1). What is your diagnosis? What is the significance of her postmenopausal bleeding?
FIGURE 17-1
Granulosa cell tumor of the ovary. (A) Ovarian gonadal stromal cells resembling the granulosa cell layer of the developing ovarian follicle are shown here forming Call-Exner bodies (microfollicular spaces filled with pale, eosinophilic material). (B) Nuclear grooves (“coffee bean nuclei”) are characteristic (high-power view). (C) Immunohistochemical expression of inhibin.
CASE 17-2
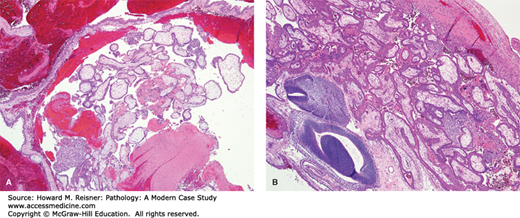
A 30-year-old woman presents to the emergency department with left-lower quadrant abdominal pain. She admits to being sexually active, but has an intrauterine device. A urinary human chorionic gonadotropin (hCG) test is positive, and an ultrasound shows a mass in the left fallopian tube. A section from the intratubal mass is shown (Figure 17-2). What is your diagnosis? What about this patient’s history made you suspect this condition? What are the clinical implications?
FIGURE 17-2
Tubal ectopic pregnancy. (A) Products of conception and blood are noted within this dilated segment of fallopian tube (low-power view). (B) At high power view fetal parts are seen, evidenced by embryonic neural tube and embryonic mesenchyme (lower left); the immature chorionic villi are shown in the top right.
The diagnosis for Case 17-1 is adult granulosa cell tumor. Figure 17-1 depicts classic histology: neoplastic cells arranged in sheets with characteristic focal gland-like structures arranged around acellular pink material, known as Call-Exner bodies. The positive immunohistochemical staining confirms via immunofixation that this tumor overexpresses inhibin, which corresponds to the elevated blood level. The granulosa cell is a normal component of the ovarian follicle, which supports the follicle. Their classification will be discussed under tumors of the ovary.
The presentation of this patient with postmenopausal bleeding highlights one of the important clinical implications of this tumor. In addition to producing inhibin, occasionally granulosa cell tumors also produce estrogen. In a postmenopausal woman, unopposed estrogen stimulation can lead to a proliferation of the endometrial lining which can be premalignant or malignant (see section on “Endometrial Hyperplasia“), which can present with abnormal bleeding. Before operating on this patient’s ovarian mass, an endometrial biopsy would be obtained to rule out a concurrent malignancy of the endometrium.
Figure 17-2 shows an ectopic pregnancy, with placental tissue composed of immature chorionic villi, syncytiotrophoblast, and the neural tube of the fetus. This condition arises when a fetus implants at any location other than inside the uterus, with the fallopian tube being the most common site. The incidence increases the most in women who have a history of pelvic inflammatory disease (PID), although women with an intrauterine device also have an increased risk.
Most patients with this condition usually present with severe abdominal pain, which can be a sign that the ectopic pregnancy has ruptured. This can be a life-threatening condition, requiring urgent surgical intervention to prevent excessive blood loss.
EMBRYOLOGY/ADULT ANATOMY
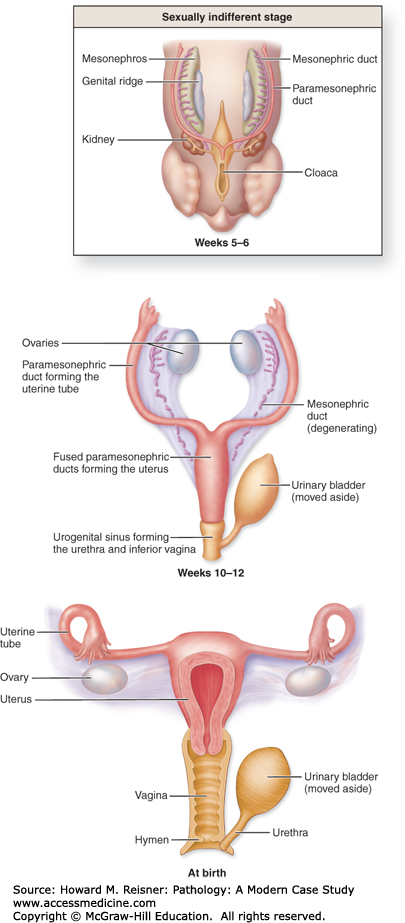
A basic knowledge of the development of the female genital tract can be extremely beneficial in the understanding of the pathogenic processes that affect these organs. Of particular importance is that fact that the ovary develops from a different cell type than the fallopian tube and uterus.
QUICK REVIEW Embryology
The germ cells that give rise to the follicles of the ovary begin in the wall of the yolk sac, and are endodermal origin. Three weeks after fertilization, these cells migrate to the urogenital ridge, where they join a proliferating group of cells, which form the supporting stroma of the ovary.
The lining of the uterus (endometrium) and muscular wall of the uterus (myometrium) are of mesodermal origin. They are formed from the fusion of the Mullerian ducts, as is the vagina. The precise origin of the cervix is still the subject of debate, but it is likely that it is also derived from Mullerian remnants.
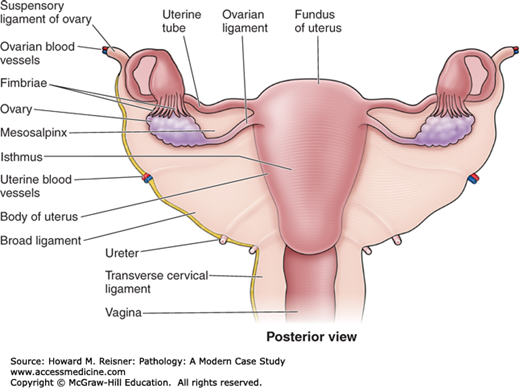
Adult AnatomyThe adult ovaries are located on either side of the uterus and are approximately 30 times the size of the newborn ovaries. They are divided into a cortex, which contains connective tissue that invests follicles at varying stages of development, and a medulla, which contains blood vessels, loosely arranged mesenchymal tissue, and remnants of ovarian development called hilar cells.
In the prepubertal female, the majority of the uterus is composed of cervix. The size and proportions of the adult uterus vary with size and the number of pregnancies (parity). The uterus is divided into three sections. The isthmus begins just above the cervix and is contiguous with the corpus (body). The fundus is the portion of the uterus above the fallopian tubes. The uterus is located between the bladder (anteriorly) and the rectum (posteriorly). It is supported by the round ligaments and the utero-ovarian ligaments, and is invested in a layer of pelvic peritoneum.
The fallopian tubes are hollow epithelial-lined muscular tubes that merge with the uterine cavity on one end and terminate into a patent end, which empties into the peritoneum. The portion closest to, and contiguous with, the uterus, is called the isthmus. The distal portion, termed ampulla, merges with the dilated end, termed fimbriae.
The anatomy of the external genital tract will be discussed in the appropriate sections below (Figures 17-3 and 17-4).
INFECTIONS
The presentation of the patient in Case 17-3 is common, as the majority of Chlamydial infections are asymptomatic. Routine screening for this disease in sexually active women is important due to the potentially grave complications of untreated infections. While some patients have only mild side effects, like urethritis and cervicitis, others may go on to develop PID. PID represents spread of an infection from the lower to the upper reproductive tract, and can lead to life-threatening illness, scarring, and infertility.
CASE 17-3
A 20-year-old sexually active woman presents to her family physician for a refill of her oral contraceptive pills (OCPs). She has had two sexual partners in the past year, and has used condoms inconsistently. Her physical exam is unremarkable. As part of her annual exam, testing for Neisseria gonorrhoeae and Chlamydia trachomatis are performed, the latter of which returns a positive result. Is it common for this disease to present in this way? What are the implications of this infection for the patient?
Fitz-Hugh–Curtis syndrome is a less common sequela of PID. This syndrome represents spread of the infection to the space around the liver and to the liver capsule (Glisson’s capsule; perihepatitis), which may result in perihepatic adhesions. Perihepatic inflammation will present in women as right-upper quadrant pain, and may be confused with more common causes of pain in that location like cholecystitis.
CASE 17-4
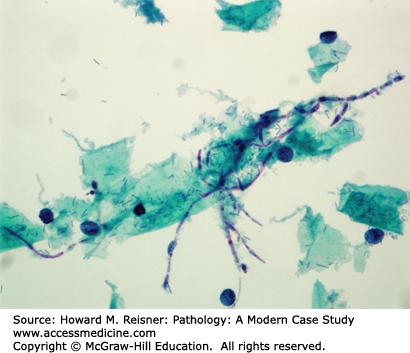
A 30-year-old woman calls her gynecologist complaining of intense vaginal itching and whitish discharge of 2 days duration. She has been on antibiotic therapy for 10 days for recent bronchitis. A pap smear, collected the day before she called the office, showed the organism pictured below (Figure 17-5). What is your diagnosis?
The organism in Figure 17-5 is Candida albicans, a yeast which is a normal part of the vaginal milieu. Symptomatic candidiasis results when a disturbance of the normal flora permits the overgrowth of this yeast, resulting in vaginal and vulvar redness, itching, and inflammation. A few conditions associated with increased incidence of yeast infection are: diabetes, recent antibiotic therapy, and pregnancy.
QUICK REVIEW
For a summary of other common sexually transmitted and genital infections, see Table 17-1.
| Infectious Organism | Characteristics | Presentation | Diagnosis |
|---|---|---|---|
| Calymmatobacterium granulomatis | Gram-negative coccobacillus that causes granuloma inguinale Rarely seen in United States, more common in tropical climates | Genital papules that develop into ulcers, heal with scarring | Identification of Donovan bodies (macrophages that have ingested the organism) on tissue section, also PCR assays now available |
| Candida albicans | Yeasts and pseudohyphae that are part of the normal vaginal flora Common infectious agent, especially in diabetics and those on antibiotic therapy | Pruritic vaginitis with white discharge and erythematous mucosa | Direct visualization from a clinical sample, occasionally empirically treated based on symptoms |
| Chlamydia trachomatis | Most common sexually transmitted infection in women and men | Urethritis, cervicitis, pelvic inflammatory disease, FHC syndrome | Preferred method: detection of Chlamydia-specific DNA in urine or from a swab of affected area |
| Gardnerella vaginalis | Gram-negative rod causes bacterial vaginosis “clue cell”—squamous cell covered in organisms | Malodorous vaginal discharge, rise in vaginal pH (>5.5) | Direct visualization from a clinical sample, occasionally empirically treated based on symptoms |
| Haemophilus ducreyi | Gram-negative rod that causes chancroid | Painful ulcers in the genital and perianal areas, enlarged inguinal lymph nodes | Clinical symptoms, culture is available |
| Herpes simplex virus | Sexually transmitted virus that infects and then remains latent in sensory ganglia, can cause recurrent outbreaks | Vesicles that ulcerate on the vulva, cervix, or perianal area If during pregnancy, delivery by cesarean to prevent infection of fetus | PCR of a swab from the center of the ulcer Can also screen for past infection with blood antibodies |
| Human Papilloma virus | Sexually transmitted virus that can lead to genital warts, or dysplasia and cancer of the cervix | Low-risk types—genital warts High-risk types—cervical dysplasia, cancer (see section on “Cervix” below) | Clinical symptoms, on Pap smear by changes in the cellular appearance, or by PCR on Pap specimen |
| Neisseria gonorrhoeae | Common gram-negative diplococci, sexually transmitted | Pelvic inflammatory disease with involvement of tubes (salpingitis) and ovaries, scarring that can lead to infertility, FHC syndrome | Preferred method: detection of Chlamydia-specific DNA in urine or from a swab of affected area |
| Treponema pallidum | Sexually transmitted spirochete that causes syphilis, progresses when left untreated | Primary—solitary, painless chancre Secondary—maculopapular rash on palms, soles, and trunk Tertiary—neurosyphilis, gummas, and aortitis | First test: nonspecific (VDRL, RPR) Second test: confirmatory (FTA-ABS) |
| Trichomonas vaginalis | Sexually transmitted flagellated protozoan with tumbling motility | Vaginitis, urethritis, “strawberry-colored” cervix, greenish vaginal discharge | Direct visualization from a clinical sample, occasionally empirically treated based on symptoms |
VULVA
The external female genitalia consists superiorly of the mons pubis and clitoris, laterally of the labia majora and more medially of the labia minora. The posterior aspect, referred to as the posterior fourchette, abuts the perineum. The opening to the vagina is termed the vestibule. Bilateral and named glands in the area consist of Skene glands (also known as periurethral glands), which open just lateral to the urethral meatus, and Bartholin glands, present laterally, which empty via ducts that drain into the posterior aspect of the vestibule.
Clitoromegaly at birth suggests an abnormality of testosterone production, which may be caused by a number of conditions, including adrenogenital syndrome and exogenous maternal ingestion.
A common benign lesion in this part of the body, the Bartholin cyst, actually represents the accumulation of secretions from the corresponding glands due to plugging of duct outflow, and characteristically presents with a palpable mass located in the lateral aspect of the vestibular orifice. Although they may recur, these cysts are benign, and may require excision when draining alone is not curative. A common cause of infection in a Bartholin gland cyst is N. gonorrhoeae.
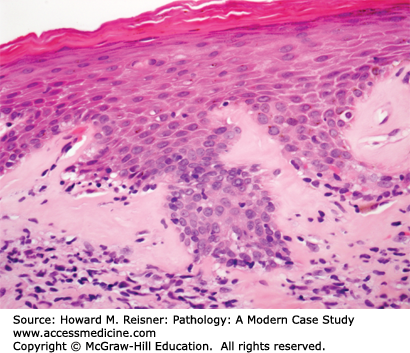
The diagnosis associated with Figure 17-6 is lichen sclerosus et atrophicus. This is an inflammatory skin disorder that is more common in postmenopausal women. It is characterized by all of the physical exam findings present in this patient. Microscopically, the epidermis is thinned by almost complete loss of the basal layer, with loss of rete pegs, and superficial hyperkeratosis (Figure 17-6). The superficial dermis is characterized by homogenization and edema, often with a band of pink, subepithelial collagen. There may be chronic inflammatory cells at the junction of the dermis and epidermis. Although it is not considered a premalignant lesion, patients with lichen sclerosus have a slightly increased risk of developing squamous cell carcinoma.
CASE 17-5
A 72-year-old woman presents to her gynecologist with a long-standing history of itching and discomfort of the labia majora and minora. Physical exam shows the affected areas of the vulva to be pale, with white plaque-like areas, thinned “parchment-like” skin, and focally, ulcerated patches. A skin biopsy is performed (Figure 17-6). What is your diagnosis? What are the implications for the patient?
Inflammatory skin disorders that affect other areas of the body can also affect the vulva, including lichen planus, psoriasis, seborrheic dermatitis, and spongiotic dermatitis. For a more thorough discussion of these diseases, please see Chapter 20.
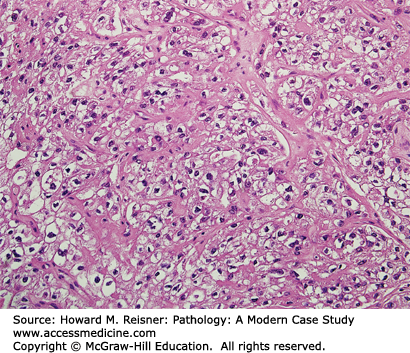
The diagnosis of the biopsy illustrated in Figure 17-7 is vulvar intraepithelial neoplasia (VIN), which represents a spectrum of dysplasia from low grade (VIN1) to high grade (VIN3). Changes in the squamous cells include: increased nuclear to cytoplasmic ratios, nuclear pleomorphism, and a lack of maturation as the cells move toward the surface. Because the nuclear abnormalities are confined to the lower third in this patient’s epithelium, this represents VIN1. The diagnosis of VIN2 is used when the abnormalities are confined to the lower two thirds, and when they are fully thick, VIN3.
There are three types of VIN. The first two: warty and basaloid, are associated with high-risk human papillomavirus (HPV) infection, most commonly HPV 16. This is the type of VIN present in this patient (pictured in Figure 17-7). Many women with these types of VIN have concurrent neoplasia of the genital tract, making this patient’s history of cervical dysplasia (also caused by infection with high-risk HPV) not surprising.
CASE 17-6
A 45-year-old woman presents to her gynecologist with the chief complaint of vulvar irritation and pruritus. She has a past medical history significant for cervical dysplasia. Physical exam shows white papules on the labia majora. A biopsy of these lesions is performed (Figure 17-7). What is your diagnosis? What are the risk factors for this condition? What serious conditions can follow as a consequence?
CASE 17-7
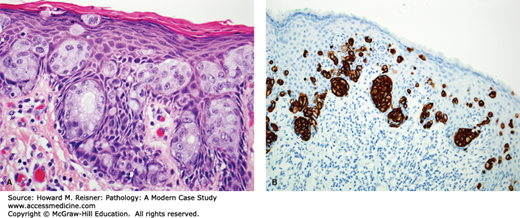
A 70-year-old woman presents to her gynecologist with the chief complaint of vulvar pruritus and erythema which had existed for some time, and which did not resolve with a variety of topical medications. She has no significant past medical history. Physical exam shows an eczematous, slightly raised erythematous area of the labia majora with focal whitish areas. A biopsy is performed (Figure 17-8). Immunohistochemical staining is performed to confirm the origin of the neoplastic cells. What is your diagnosis? For what other conditions must you screen this patient?
The third type of VIN is termed differentiated VIN (or VIN, simplex type), and while not associated with HPV infections, its incidence is increased in women with inflammatory dermatitis of the vulva, most notable lichen sclerosus. Though their causative agents are not the same, all three subtypes of VIN represent precursor lesions for vulvar squamous cell carcinoma.
The biopsy illustrated in Figure 17-8 demonstrates extramammary Paget disease. The Paget cells are distinct from surrounding epithelium, with paler cytoplasm and more prominent nuclei. They are present in large numbers at the junction of the epidermis and dermis, sometimes in nests and rarely small glands, and other times as single cells. Paget disease can present as a solitary finding of primary cutaneous origin (primary cutaneous Paget disease), or may be a manifestation of an underlying malignancy of the surrounding skin, the anal–rectal region, or the bladder (secondary Paget disease or Paget disease of noncutaneous origin).
Microscopically, the cells of Paget disease mimic those of a cutaneous malignancy, which can present in this part of the body, malignant melanoma. Immunohistochemistry can help to differentiate between these two entities. As shown in Figure 17-6, the neoplastic cells are epithelial in origin, staining positively with low molecular weight cytokeratins CK7 and CAM5.2, and do not stain for the melanoma markers, S100, HMB-45, and MART-1.
VAGINA
Developmental abnormalities include a septate (doubled) vagina, which is often accompanied by a duplicate uterus (uterus didelphys).
Infectious organism of the external genitalia often affects the vagina. For details, see Table 17-1. In addition, systemic inflammatory diseases, such as Crohn disease and Stevens–Johnson syndrome, may also affect the vagina.
The most common malignant tumor of the vagina is metastatic cervical carcinoma. Although primary squamous cell carcinoma of the vagina is rare when compared to cervical and vulvar carcinoma, it does occur. Like vulvar carcinoma, it is preceded by three levels of dysplasia, and uses the same criteria to differentiate between the grades of vaginal intraepithelial neoplasia. Risk factors include HPV infection, immunosuppression, and a history of ionizing radiation.
HUNTING FOR ZEBRAS
Clear Cell Carcinoma of the Vagina: Originally, a rare diagnosis made in postmenopausal women, from 1970 to 1990, it was noted that cases of clear cell carcinoma were being diagnosed in much younger women (mean age of 19). The cause was linked to intrauterine exposure to diethylstilbestrol, a drug used by many pregnant women from 1940 to 1970 to prevent miscarriage. These tumors, identical to their counterparts in the ovary and endometrium, show cells with prominent cytoplasmic clearing and/or cytoplasmic eosinophilic cell change forming a solid and/or tubulocystic patterns, with the small tubules and cysts lined by “hobnail” cells (Figure 17-9). It seems that the cohort of exposed females have now aged, and the vast majority of new cases are once again in postmenopausal women.
IN TRANSLATION
Embryonal Rhabdomyosarcoma: Representing the most common primary vaginal neoplasm in infants and children, the malignant cell of origin in this tumor is skeletal muscle. The most common subtype, called sarcoma botryoides, is characterized by a “cambium layer” of condensed rhabdomyoblasts beneath the epithelium (Figure 17-10). When the tumor mass is large, some patients will present with the classic “cluster of grapes” protruding from the vaginal orifice.
Embryonal rhabdomyosarcoma is frequently associated with loss of heterozygosity in a region of the short arm of chromosome 11 (11p15.5), which is rich in imprinted genes. This could potentially lead to inactivation of a tumor suppressor gene or activation of a proto-oncogene resulting in neoplastic progression. Defects in this chromosomal region are also associated with Beckwith–Wiedemann syndrome (BWS). Children with BWS have an over 500-fold risk for developing several embryonal tumors, such as Wilms tumor and hepatoblastoma.
FIGURE 17-10
Sarcoma botryoides (embryonal rhabdomyosarcoma, botryoid type). (A) An edematous, grape-like structure is noted in this tumor (low-power view). (B) At high-power view, the surface of the tumor shows benign glandular epithelium with periglandular stromal condensation by the malignant mesenchymal component (the so-called “cambium layer”). (C) This field is entirely represented by sheets of embryonal rhabdomyosarcoma with a very undifferentiated appearance.
CERVIX
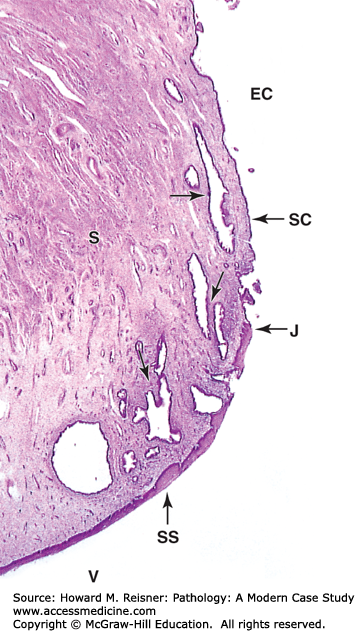
The uterine cervix consists of the endocervix, which is contiguous with the uterus, and the outer portion that opens into the vagina, the exocervix. The patent center of the cervix is called the os. The endocervix is lined by mucous-secreting cells, while the exocervix is lined by squamous epithelium. The area where these two meet is called the transformation zone. This zone is pathologically important, as it is the preferred locale for infection by HPV, a known precursor of cervical dysplasia and carcinoma. The transformation zone migrates throughout life as the cervix elongates (Figures 17-4 and 17-11).
FIGURE 17-11
Cervix. The cervix is the lower part of the uterus, which extends into the upper vagina. Micrograph shows that the mucosa of the endocervical canal (EC) is continuous with the endometrium and like that tissue is lined by simple columnar epithelium (SC). The endocervical mucosa has folds and many large branched cervical glands (arrows) secreting mucus under the influence of the ovarian hormones and often becoming quite dilated. At the external os, the point at which this canal opens into the vagina (V), there is an abrupt junction (J) between this simple epithelium and the stratified squamous epithelium (SS) covering the exocervix and vagina. The junctional region defines the transformation zone ×15. H&E. (Modified from Mescher AL, Junqueira’s Basic Histology Text and Atlas. 12th edn. McGraw Hill Lange New York, Figure 22-21.)
Inflammation: The normal flora of the vagina is dominated by Lactobacilli, which produce acid and maintain the vaginal pH below 4.5. This environment curbs the growth of other organisms, like yeast. However, normal physiologic events, like menstruation, can alter the vaginal pH and result in shifts in vaginal flora. These normal changes lead most women to show signs of inflammation in the cervix. Marked or destructive inflammation, however, must be noted on cervical biopsies, as it may be the harbinger of a more serious upper tract infection like Chlamydia, Herpes Simplex Virus, or gonococcus (see Table 17-1).
Benign Lesions of Cervix: Two benign lesions of the cervix are noted here due to their potential confusion for a malignant condition. Leiomyomas are benign, circumscribed tumors of smooth muscle. Although they occur most frequently in the uterus, cervical leiomyomas can cause reactive atypia of the overlying epithelium. Cervical polyps are relatively common exophytic growths that occur in adult women. Because they can present with vaginal bleeding and a mass, they occasionally cause clinical concern. However, simple excision shows them to be benign fibrous tissue covered by columnar epithelium. Occasionally, entrapped mucous-filled spaces, termed, Nabothian cysts, may present as a cervical polyp as well.
Squamous Cell Lesions: The implementation of screening for squamous cell carcinoma of the cervix is an important success story in the history of medicine. Early detection of precursor lesions by Pap smear (named after its creator, Papanicolaou), allows for clinical intervention before carcinoma develops in the vast majority of cases. Once a relatively common condition, invasive squamous cell carcinoma has been decreasing in incidence in the West for some time as a result, although it continues to be a major public health problem in less developed countries throughout the world.
The pathogenesis of cervical carcinoma has been linked to infection with HPV, a DNA virus that has many subtypes. There are known types that are “high risk” for causing cervical carcinoma, namely, HPV 16 and 18, and others that are “low risk,” and tend to cause lesions like genital warts, namely, HPV 6 and 11. Risk factors that increase exposure to HPV infections also increase an individual’s likelihood of developing cervical dysplasia and cancer. Among these are: early age at first intercourse, multiple sexual partners or a male partner with multiple previous partners, persistent infection with high-risk HPV subtypes, and high parity. Other risk factors, like immunosuppression and smoking, compromise the body’s defense against the infecting virus.
HPV infection, even with high-risk serotypes, can be transient. It is known that younger women more often clear the virus. It is, therefore, essential for the treating clinician to take into account a woman’s age when making management decisions for patients with cervical dysplasia. The general trend is to manage younger patients less aggressively, and older patients more vigilantly.
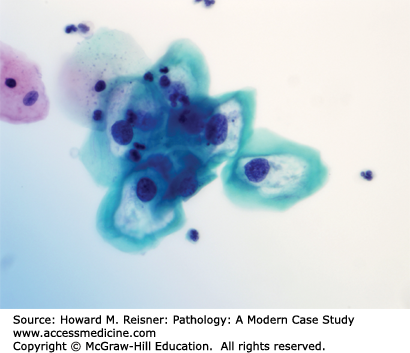
The Pap smear in Figure 17-12 shows benign squamous cells with pink cytoplasm and more atypical cells with blue cytoplasm, called koilocytes. This term is used for squamous cells that show changes due to HPV infection. In addition to the perinuclear halo (seen above as a clear space around the nucleus), nuclear changes present in the koilocytes include enlargement, irregular borders, and clumping of chromatin. The diagnosis in this patient is low-grade squamous intraepithelial lesion.
FIGURE 17-12
Low-grade squamous intraepithelial lesion (LGSIL) of the cervix. Dysplastic squamous epithelial cells (located centrally) show twofold nuclear enlargement compared to the small nuclei of normal squames (present at top left); perinuclear haloes present in the dysplastic cells signify accompanying koilocytosis (HPV viral cytopathic effect).
CASE 17-8
A 26-year-old woman with no past medical history presents to her gynecologist for her annual exam. She has had six lifetime sexual partners. Her Pap smear is shown in Figure 17-12. What is your diagnosis?
The results of her Pap smear prompts cervical biopsies, one of which is pictured in Figure 17-13. What is your diagnosis? Why was a biopsy performed?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree