ANATOMIC AND HISTOLOGIC RECALL
The eye is located within the bony structure of the skull, the orbit. The eye is connected to the brain by the optic nerve and to the orbit by a number of striated muscles that control its movements. Besides the eye and the muscles, the orbit is filled with loose connective tissue that contains nerves, vessels, and small deposits of lymphocytes.
The anterior surface of the eye, a transparent to light lens-like structure, the
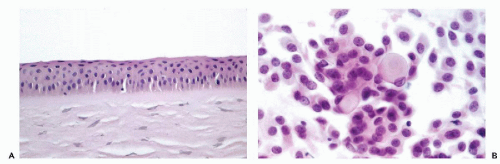
cornea, is lined on its surface by a transparent,
stratified, nonkeratinized squamous epithelium (
Fig. 41-1A). Laterally, the cornea becomes the
sclera, a fibrous structure that encloses the eye. The eye is protected anteriorly by the
eyelids which, on the surface facing the eye, are lined by a stratified epithelium containing numerous
mucus-producing goblet cells. This epithelium is in continuity with the squamous epithelium lining the cornea; the transition occurs at the
limbus, where the peripheral cornea merges with the anterior sclera. The outer surface of the eyelids is formed by skin. The eyelids contain numerous
mucus-producing glands, the largest and most important being the
meibomian glands. Smaller glands are known as glands of Zeis and Moll. Extending from the eyelids into the orbit are the
lacrimal glands, similar in structure to
serous salivary glands, which, by a series of canals, secrete
tears that lubricate the eye and the eyelids.
The
internal structure of the eye is extremely complex and beyond the scope of this chapter, thus only the key structures will be mentioned. The interior of the eye is divided into two chambers: the
anterior aqueous chamber, located between the cornea and the transparent crystalline lens, and the
posterior chamber, filled with transparent viscous vitreous (vitreous body), demarcated anteriorly by the lens and posteriorly by the retina. The anterior chamber contains a contractile pigmented structure, the
iris, forming the pupil of the eye and regulating the input of light. All parts of the eye serve the primary purpose of processing light signals by multilayered sensory complex neuronal tissue, the
retina. The retina can become the site of a malignant tumor of childhood, the
retinoblastoma. On the outer, orbital side, the retina is supported by a layer of
melanin-containing pigment epithelium that extends anteriorly into the iris. In turn, the pigmented epithelium is separated from the sclera by an intermediate layer, the
uvea, composed
of connective tissue containing blood vessels, nerves, and melanocytes. The uvea is divided into three distinct anatomic segments: the
choroid, which surrounds most of the eye and transits anteriorly into the
ciliary body, and the
iris. The most common malignant tumors of the eye,
malignant melanomas, develop in the uvea, particularly in the
choroid but also, less commonly, in the
ciliary body and the
iris. The
optic nerve may be the site of formation of orbital
gliomas and meningiomas. For a detailed description of the histology of the eye, the reader is referred to a simple, yet detailed and accurate account by
Stevens and Lowe (1992).