Chapter 4 The Eye
A. Generalities
2 What is needed to measure visual acuity?
Snellen chart, pinhole occluder, and pocket-size near-vision test card. All are readily available.
10 How is vision measured in illiterate patients or children?
By using the single “E” chart and asking patients to designate the strokes’ direction.
11 How is vision measured in bedridden patients?
By using the Rosenbaum card, which can be found at any medical supply store.
12 What is the significance of reduced visual acuity?
It may indicate any of the following ocular processes:
 Refractive and correctable errors (myopia, astigmatism, and presbyopia)
Refractive and correctable errors (myopia, astigmatism, and presbyopia)
 Treatable and reversible blinding disease (cataracts or uveitis)
Treatable and reversible blinding disease (cataracts or uveitis)
 Manifestations of systemic disorders, progressive if untreated (diabetes or hypertension)
Manifestations of systemic disorders, progressive if untreated (diabetes or hypertension)
 Vision-impairing and possibly life-threatening CNS disease (multiple sclerosis, gliomas)
Vision-impairing and possibly life-threatening CNS disease (multiple sclerosis, gliomas)
 Congenital disorders (rubella or toxoplasmosis)
Congenital disorders (rubella or toxoplasmosis)
 Infectious diseases (cytomegalovirus, retinitis, or toxoplasmosis)
Infectious diseases (cytomegalovirus, retinitis, or toxoplasmosis)
13 How can one confirm the cause of reduced visual acuity?
 Uncorrected refractive errors of the eye are usually suspected whenever vision improves with a pinhole occluder.
Uncorrected refractive errors of the eye are usually suspected whenever vision improves with a pinhole occluder.
 Opacities of the light-transmitting media are diagnosed by either ophthalmoscopy or by simply examining the red reflex.
Opacities of the light-transmitting media are diagnosed by either ophthalmoscopy or by simply examining the red reflex.
 Neurologic or retinal disorders are revealed by ophthalmoscopy, the swinging flashlight test, or visual field testing.
Neurologic or retinal disorders are revealed by ophthalmoscopy, the swinging flashlight test, or visual field testing.
 Amblyopia is suggested by a childhood history of reduced visual acuity often accompanied by strabismus.
Amblyopia is suggested by a childhood history of reduced visual acuity often accompanied by strabismus.
15 When should you refer a patient with subnormal visual acuity?
 No symptoms, but visual acuity of 20/40 (or worse) in one or both eyes
No symptoms, but visual acuity of 20/40 (or worse) in one or both eyes
 A visual acuity difference between eyes of two lines or more
A visual acuity difference between eyes of two lines or more
 Middle-aged or elderly patients with presbyopia, even if distance visual acuity is preserved (for prescription of reading glasses)
Middle-aged or elderly patients with presbyopia, even if distance visual acuity is preserved (for prescription of reading glasses)
 Presence of an afferent pupillary defect
Presence of an afferent pupillary defect
B. Color Vision
C. Visual Fields
21 Describe the normal anatomy of the visual pathways.
This is quite complex and yet fundamental for the understanding of visual field defects (Fig. 4-1). Our visual world (i.e., visual field) is divided into right and left hemifields. Each eye gets information from both.
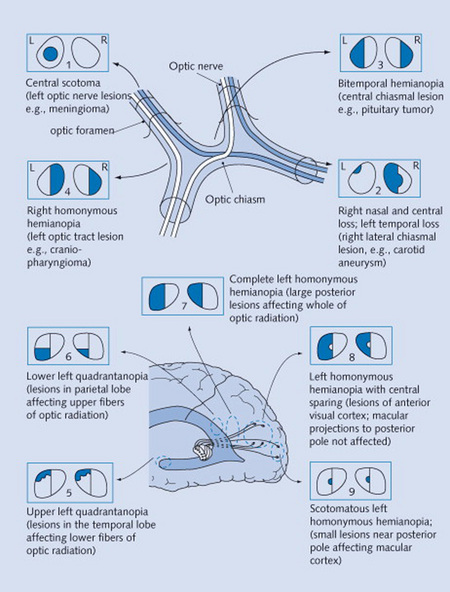
Figure 4-1 Neurologic visual pathways, and diseases originating from their interruption.
(From Mihailoff G: Crash Course Nervous System. St. Louis, Mosby, 2005.)
22 How do you test visual fields?
 Static testing: The examiner sits 3 feet away from the patient, who covers one eye with the palm of one hand while fixating on the examiner’s opposite eye. While the patient is doing so, the examiner outstretches an arm and briefly holds up one or two fingers. These are then displayed in each quadrant, and the patient is asked to report how many fingers are seen. In fact, the maneuver is often carried out by testing two quadrants simultaneously (by presenting both examiner’s hands to the two quadrants at the same time). This may unmask a parietal lesion that would otherwise allow a patient to see a single object (but not a double) by relying on the contralateral field. The test is then repeated for the opposite eye.
Static testing: The examiner sits 3 feet away from the patient, who covers one eye with the palm of one hand while fixating on the examiner’s opposite eye. While the patient is doing so, the examiner outstretches an arm and briefly holds up one or two fingers. These are then displayed in each quadrant, and the patient is asked to report how many fingers are seen. In fact, the maneuver is often carried out by testing two quadrants simultaneously (by presenting both examiner’s hands to the two quadrants at the same time). This may unmask a parietal lesion that would otherwise allow a patient to see a single object (but not a double) by relying on the contralateral field. The test is then repeated for the opposite eye.
 Dynamic testing: This is carried out very much like its static counterpart, except that the examiner continuously wiggles his or her fingers while gradually (and very slowly) bringing them into the patient’s visual field from a peripheral angle. The patient is then asked to report when (s)he sees the fingers.
Dynamic testing: This is carried out very much like its static counterpart, except that the examiner continuously wiggles his or her fingers while gradually (and very slowly) bringing them into the patient’s visual field from a peripheral angle. The patient is then asked to report when (s)he sees the fingers.
25 What is the difference between anterior and posterior visual field defects?
 Anterior defects are unilateral (monocular)—typically crossing the vertical meridian.
Anterior defects are unilateral (monocular)—typically crossing the vertical meridian.
 Posterior defects are instead bilateral, with borders aligned to (and not crossing) the vertical meridian of the visual field. They are referred to as chiasmal if the defect is bitemporal and retrochiasmal if homonymous (see later).
Posterior defects are instead bilateral, with borders aligned to (and not crossing) the vertical meridian of the visual field. They are referred to as chiasmal if the defect is bitemporal and retrochiasmal if homonymous (see later).
26 What are the causes of visual field defects?
 Prechiasmal defects are due to ocular lesions (glaucoma, retinal emboli, optic neuritis).
Prechiasmal defects are due to ocular lesions (glaucoma, retinal emboli, optic neuritis).
 Chiasmal defects are due to pituitary lesions (mostly neoplastic).
Chiasmal defects are due to pituitary lesions (mostly neoplastic).
 Postchiasmal defects are due to cortical lesions (temporal, parietal, or occipital).
Postchiasmal defects are due to cortical lesions (temporal, parietal, or occipital).
 Optic tracts lesions are instead rare, representing only 5% of all postchiasmal defects.
Optic tracts lesions are instead rare, representing only 5% of all postchiasmal defects.
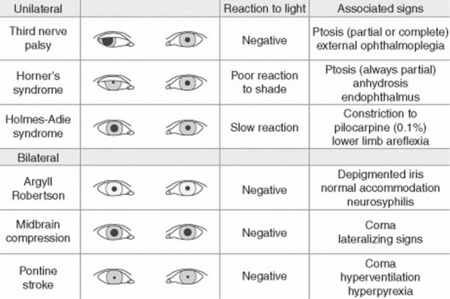
D. Pupils
37 Why is examination of the pupils important?
Because attention to pupillary shape, size, and response to external stimuli provides extremely valuable clinical information. Figures 4-2 and 4-3 summarize the most common pupil abnormalities.
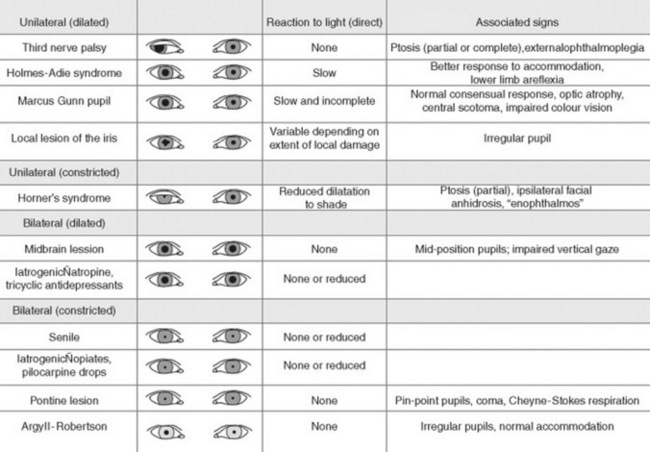
Figure 4-2 Pupil abnormalities.
(From Liporace J: Crash Course Neurology. St. Louis, Mosby, 2006, Fig. 6.3.)
44 What are the most common abnormalities in pupillary shape?
 The most common is probably the irregular, pear-shaped contour of pupils that have undergone an intraocular surgical procedure, such as cataract excision. There also are other causes of oval pupils, such as an incipient transtentorial uncal herniation (before the pupil becomes dilated, fixed, and fully round) and Adie’s pupil.
The most common is probably the irregular, pear-shaped contour of pupils that have undergone an intraocular surgical procedure, such as cataract excision. There also are other causes of oval pupils, such as an incipient transtentorial uncal herniation (before the pupil becomes dilated, fixed, and fully round) and Adie’s pupil.
 Blunt trauma to the eye. This may tear the iris sphincter, making the affected pupil larger and slightly irregular.
Blunt trauma to the eye. This may tear the iris sphincter, making the affected pupil larger and slightly irregular.
 Inflammation of the iris (iritis). This may cause adhesions (synechiae) of the iris to the anterior capsule of the lens, thus making the pupil irregular.
Inflammation of the iris (iritis). This may cause adhesions (synechiae) of the iris to the anterior capsule of the lens, thus making the pupil irregular.
 A coloboma of the iris. This is a congenital defect characterized by incomplete closure of the embryonic fissure of the optic cup. The involved iris usually has a keyhole shape, with the defect being most commonly in an inferonasal position.
A coloboma of the iris. This is a congenital defect characterized by incomplete closure of the embryonic fissure of the optic cup. The involved iris usually has a keyhole shape, with the defect being most commonly in an inferonasal position.
47 What are the most common causes of anisocoria?
 Simple (physiologic) anisocoria: A normal variant characterized by a physiologic difference of at least 0.4 mm between the two pupils, due, in turn, to an imbalance in muscular tone of the right and left sphincters. It is the most common anisocoria, present at all times in 3% of the population and at some times in up to 20%, with presence or absence depending on day of observation. In contrast to pathologic anisocoria, the physiologic form is characterized by a pupillary difference that does not change with various levels of illumination. Moreover, physiologic anisocoria is chronic, rarely >1 mm and always isolated (i.e., never associated with ptosis double vision or light-near dissociation see later). Hence, presence of concomitant findings makes anisocoria a much more ominous condition.
Simple (physiologic) anisocoria: A normal variant characterized by a physiologic difference of at least 0.4 mm between the two pupils, due, in turn, to an imbalance in muscular tone of the right and left sphincters. It is the most common anisocoria, present at all times in 3% of the population and at some times in up to 20%, with presence or absence depending on day of observation. In contrast to pathologic anisocoria, the physiologic form is characterized by a pupillary difference that does not change with various levels of illumination. Moreover, physiologic anisocoria is chronic, rarely >1 mm and always isolated (i.e., never associated with ptosis double vision or light-near dissociation see later). Hence, presence of concomitant findings makes anisocoria a much more ominous condition.
 Pharmacologic dilation: Another common and benign cause. This can be due to conscious or inadvertent instillation of mydriatic drops (like in Italian Renaissance women) or even to improper nebulization of anticholinergic agents. The latter may create a diagnostic dilemma in intensive care unit patients, whose mental status often waxes and wanes. A response to cholinergic eye drops (such as pilocarpine) may help separating pharmacologic paralysis (which will remain unresponsive) from either Adie’s pupil or a third nerve palsy (which, instead, will constrict in response to topical cholinergics—previously discussed).
Pharmacologic dilation: Another common and benign cause. This can be due to conscious or inadvertent instillation of mydriatic drops (like in Italian Renaissance women) or even to improper nebulization of anticholinergic agents. The latter may create a diagnostic dilemma in intensive care unit patients, whose mental status often waxes and wanes. A response to cholinergic eye drops (such as pilocarpine) may help separating pharmacologic paralysis (which will remain unresponsive) from either Adie’s pupil or a third nerve palsy (which, instead, will constrict in response to topical cholinergics—previously discussed).
 Third cranial nerve palsy (i.e., parasympathetic denervation): This is characterized by (1) ipsilateral mydriasis plus; (2) ptosis (from paralysis of the levator palpebrae); and (3) weakness of all extraocular muscles, with the exception of the lateral rectus and superior oblique (which are the only ones not controlled by the third cranial nerve). Hence, the affected eye will be deviated outward and downward, thus resulting in diplopia. Note that the dilated pupil will still constrict if a cholinergic drop is instilled in the eye. Moreover, due to defective constriction, the anisocoria of third-nerve palsy is greater in bright illumination.
Third cranial nerve palsy (i.e., parasympathetic denervation): This is characterized by (1) ipsilateral mydriasis plus; (2) ptosis (from paralysis of the levator palpebrae); and (3) weakness of all extraocular muscles, with the exception of the lateral rectus and superior oblique (which are the only ones not controlled by the third cranial nerve). Hence, the affected eye will be deviated outward and downward, thus resulting in diplopia. Note that the dilated pupil will still constrict if a cholinergic drop is instilled in the eye. Moreover, due to defective constriction, the anisocoria of third-nerve palsy is greater in bright illumination.
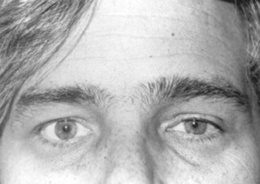
 Horner’s syndrome (Fig. 4-4): First described in 1860 by the Swiss ophthalmologist Johann Friedrich Horner, this is characterized by miosis of the affected pupil (from paralysis of the pupillodilator muscle) plus dysautonomic findings, including (1) ipsilateral ptosis (from paralysis of the superior tarsal muscle), and (2) anhidrosis (from damage to sudomotor fibers). In contrast to physiologic anisocoria, the difference between pupils in Horner’s varies with illumination: greater in the dark (revealing defective dilation) and smaller in bright light (demonstrating intact constriction). Response to cocaine drops is also different: Horner’s worsens, whereas simple anisocoria improves. This has strong predictive value, with sensitivity and specificity > 95%, a positive likelihood ratio (LR) of 96.8, and a negative LR of 0.1. Horner’s should always prompt a search for lesions of (1) the first-order neuron (such as a brainstem stroke, the most common cause of Horner’s on a neurology service; hence, the need for a thorough neurologic exam, with special attention to a lateral medullary syndrome); (2) the second-order neuron (usually a tumor of lung or thyroid, the most common cause of Horner’s on a medical service; hence, the need for a thorough neck/supraclavicular/respiratory exam); or, finally (3) the third-order neurons. These are less common causes of Horner’s, usually due to vascular headache (migraine), trauma or inflammation of the orbit, and cavernous sinus syndrome. Lesions of third-order neurons may preserve facial sweating.
Horner’s syndrome (Fig. 4-4): First described in 1860 by the Swiss ophthalmologist Johann Friedrich Horner, this is characterized by miosis of the affected pupil (from paralysis of the pupillodilator muscle) plus dysautonomic findings, including (1) ipsilateral ptosis (from paralysis of the superior tarsal muscle), and (2) anhidrosis (from damage to sudomotor fibers). In contrast to physiologic anisocoria, the difference between pupils in Horner’s varies with illumination: greater in the dark (revealing defective dilation) and smaller in bright light (demonstrating intact constriction). Response to cocaine drops is also different: Horner’s worsens, whereas simple anisocoria improves. This has strong predictive value, with sensitivity and specificity > 95%, a positive likelihood ratio (LR) of 96.8, and a negative LR of 0.1. Horner’s should always prompt a search for lesions of (1) the first-order neuron (such as a brainstem stroke, the most common cause of Horner’s on a neurology service; hence, the need for a thorough neurologic exam, with special attention to a lateral medullary syndrome); (2) the second-order neuron (usually a tumor of lung or thyroid, the most common cause of Horner’s on a medical service; hence, the need for a thorough neck/supraclavicular/respiratory exam); or, finally (3) the third-order neurons. These are less common causes of Horner’s, usually due to vascular headache (migraine), trauma or inflammation of the orbit, and cavernous sinus syndrome. Lesions of third-order neurons may preserve facial sweating.
 Miscellaneous: These include (1) inflammatory processes (e.g., unilateral iritis), (2) old trauma, (3) acute angle closure glaucoma, (4) various neurologic disorders, and (5) previous intraocular surgery (i.e., cataract extraction). In case of the red eye (see later), presence of anisocoria argues in favor of a more serious disease.
Miscellaneous: These include (1) inflammatory processes (e.g., unilateral iritis), (2) old trauma, (3) acute angle closure glaucoma, (4) various neurologic disorders, and (5) previous intraocular surgery (i.e., cataract extraction). In case of the red eye (see later), presence of anisocoria argues in favor of a more serious disease.