Chapter 14 Lung Auscultation
Generalities
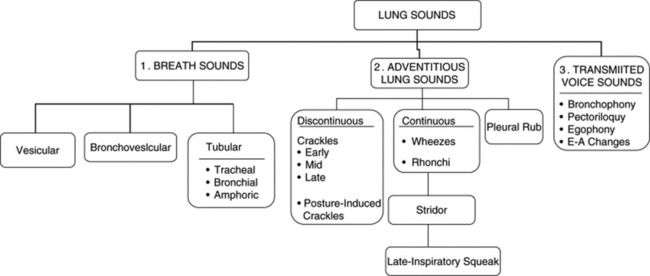
5 What are lung sounds?
They are sounds generated by the lungs. Transmitted voice sounds are instead sounds generated by the larynx and subsequently transmitted through the lungs (Fig. 14-1).
6 What are the major types of lung sounds (respiratory sounds)?
Each of these subgroups contains various other sounds (Table 14-1).
A. Breath Sounds (Basic Lung Sounds)
9 What are the major types of breath sounds?
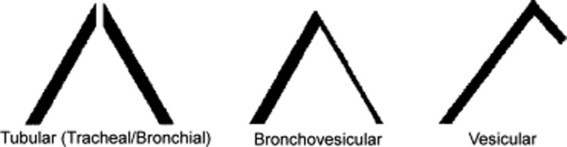
This traditional division is based on the sound’s site of production. Conversely, a more recent classification (based on physical characteristics) reports only two major lung sounds: (1) the normal ones (or vesicular breath sounds) and (2) the abnormal ones (or tubular breath sounds). The latter consist of three subvarieties: tracheal, bronchial, and amphoric. As for the bronchovesicular breath sound, it is in a league of its own and one that should probably be abandoned. (Fig. 14-2)
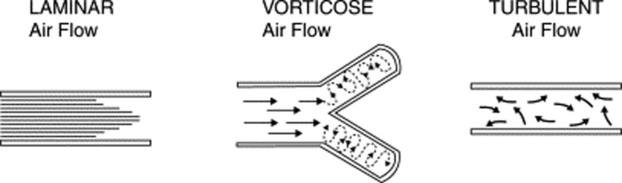
10 What is the air movement responsible for the production of breath sounds?
It depends on the size of the airway. Overall, three types of movement may take place along the tracheobronchial tree (Fig. 14-3). Some are silent and others noisy:
 Laminar airflow is characteristic of small peripheral airways. It is so slow that airflow in the alveoli almost comes to an end. Hence, this is a silent movement.
Laminar airflow is characteristic of small peripheral airways. It is so slow that airflow in the alveoli almost comes to an end. Hence, this is a silent movement.
 Vorticose airflow is a bit faster and typical of medium-sized branching airways. “Branching” separates airflow in different layers with different velocities, whose interaction generates eddies and vortices—all noisy. This air movement is often called mixed (or transitional) because it resembles both laminar and turbulent airflow.
Vorticose airflow is a bit faster and typical of medium-sized branching airways. “Branching” separates airflow in different layers with different velocities, whose interaction generates eddies and vortices—all noisy. This air movement is often called mixed (or transitional) because it resembles both laminar and turbulent airflow.
 Turbulent airflow is very rapid, complex, and typical of large central airways (trachea and major bronchi). Air molecules randomly collide against each other and onto the airway walls. This air movement is characteristically noisy.
Turbulent airflow is very rapid, complex, and typical of large central airways (trachea and major bronchi). Air molecules randomly collide against each other and onto the airway walls. This air movement is characteristically noisy.
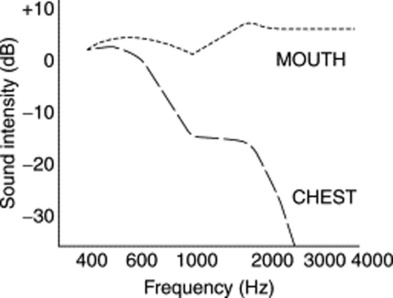
14 What are the acoustic characteristics of breath sounds at the mouth?
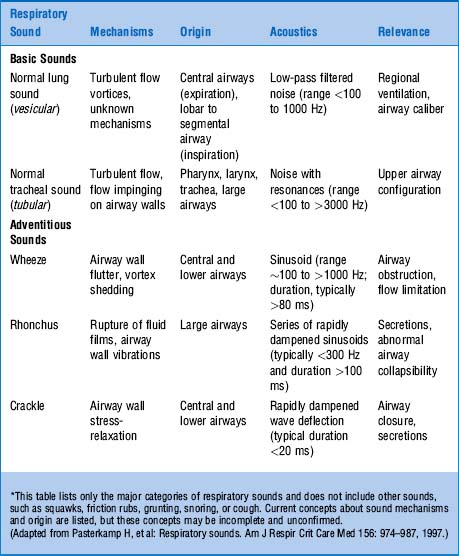
The dominant one is the high pitch (and loudness). Hence, sounds at the mouth have much wider frequencies, ranging between 200 and 2000 Hz—very much like white noise (Fig. 14-4).
17 Are there differences in intensity of breath sounds between the various types of airflow obstruction?
Yes (Table 14-2). In fact, the intensity of inspiratory breath sounds at the mouth can help differentiate emphysema from chronic bronchitis or asthma, since in only the last two conditions it directly correlates with (1) increased airway resistance; (2) reduced FEV1 (forced expiratory volume in 1 sec); and (3) reduced peak expiratory flow rate (PEFR). Conversely, in emphysema, inspiratory breath sounds at the mouth are paradoxically quiet and almost silent because emphysema causes no direct narrowing of the bronchi, but only a dynamic expiratory airflow obstruction due to loss of elastic recoil.
Table 14-2 Changes in Lung Sounds with Pulmonary Disease
| Lung Disease | Breath Sounds | Adventitious Lung Sound |
|---|---|---|
| Pneumonia | Bronchial or absent | Inspiratory crackles |
| Atelectasis | Harsh/bronchial | Late inspiratory crackles |
| Pneumothorax | Absent | None |
| Emphysema | Diminished | Early inspiratory crackles |
| Chronic bronchitis | Normal | Wheezes and crackles |
| Pulmonary fibrosis | Harsh | Inspiratory crackles |
| Congestive heart failure | Diminished | Inspiratory crackles |
| Pleural effusion | Diminished | None |
| Asthma | Diminished | Wheezes |
(From Wilkins R: Lung Sounds. St. Louis, Mosby, 1996.)
22 What are the main characteristics of tubular breath sounds?
 Loudness (graphically represented by thick inspiratory and expiratory lines)
Loudness (graphically represented by thick inspiratory and expiratory lines)
 A brief silent pause between inspiration and expiration
A brief silent pause between inspiration and expiration
 A long expiratory phase, usually as long as inspiration (I:E ratio of approximately 1:1) (Fig. 14-5)
A long expiratory phase, usually as long as inspiration (I:E ratio of approximately 1:1) (Fig. 14-5)
26 What are the three characteristics of vesicular breath sounds?
 Low frequency. Hence, soft and muffled, graphically represented by thin respiratory lines.
Low frequency. Hence, soft and muffled, graphically represented by thin respiratory lines.
 Absence of the silent pause between inspiration and expiration
Absence of the silent pause between inspiration and expiration
 Short duration of exhalation because most high frequencies are concentrated in the last two thirds of exhalation and thus totally eliminated by the presence of alveolar air.
Short duration of exhalation because most high frequencies are concentrated in the last two thirds of exhalation and thus totally eliminated by the presence of alveolar air.
37 Are vesicular breath sounds normally heard throughout the chest?
No. Although present over most lung fields of normal patients, vesicular sounds are classically absent in two narrow areas, corresponding anteriorly and posteriorly to the trachea and central bronchi (parascapular areas). Here, the vesicular sounds are replaced by bronchovesicular sounds (see also below, questions 60–62). Outside these narrow areas, presence of distinct vesicular sounds remains a sine qua non for a healthy lung (Fig. 14-6).
41 What is the best bedside predictor for the presence of chronic obstructive lung disease?
A reduction in breath sound intensity (BSI). A total of 32 findings has been said to indicate COPD, with many arguing strongly for its presence (Table 14-3), yet BSI is the single best index of emphysema. Early inspiratory crackles also argue for obstruction (LR, 14.6), but mostly chronic bronchitis. If progressive over time, BSI reduction can help monitor methacholine challenge, even when wheezing is absent. Finally, any two of the following virtually rule in airflow limitation: >70-pack-years of smoking, decreased breath sounds, or history of COPD. Years of cigarette smoking, subjective wheezing, and either objective wheezing or peak expiratory flow rate also predict the likelihood of airflow limitation in males. Although other signs have been linked to obstruction (objective wheezing, barrel chest, positive match test, rhonchi, hyperresonance, and subxiphoid apical impulse), on multivariate analysis only three remain significantly associated with its diagnosis: self-reported history of COPD (LR, 4.4), wheezing (LR, 2.9), and FET >9 seconds (LR, 4.6). Patients with all three have an LR of 33 (ruling in COPD); those with none have an LR of 0.18 (ruling out COPD).
Table 14-3 Accuracy of Bedside Findings for the Evaluation of Obstructive Lung Disease: Likelihood Ratios, Point Estimates, and 95% Confidence Intervals
| Findings | Positive LR(95% CI) | Negative LR(95% CI) |
|---|---|---|
| Subxiphoid cardiac impulse | 7.4 (2.0, 27.1) | 0.9 (0.7, 1.1) |
| Absent cardiac dullness | 11.8 (1.2, 121.4) | 0.9 (0.7, 1.1) |
| Hyperresonance | 5.1 (1.7, 15.6) | 0.7 (0.5, 1.0) |
| Diaphragm excursion <2 cm | 5.3 (0.8, 35.0) | 0.9 (0.7, 1.1) |
| Breath sound intensity <9 | 10.2 (4.6, 22.7) | – |
| Breath sound intensity 10–12 | 3.6 (1.4, 9.5) | – |
| Breath sound intensity 13–15 | 0.7 (0.3, 1.5) | – |
| Breath sound intensity >15 | 0.1 (0, 0.3) | – |
| Forced expiratory time <3 sec | 0.2 (0.1, 0.3) | – |
| Forced expiratory time 3–9 sec | 1.3 (0.5, 2.9) | – |
| Forced expiratory time >9 sec | 4.8 (1.3, 17.6) | – |
| Early crackles, detecting obstructive disease | 14.6 (3.0, 70) | 0.4 (0.1, 1.4) |
| Early crackles, detecting severe obstruction | 20.8 (3.0, 142.2) | 0.1 (0, 0.4) |
| Unforced wheezes, detecting obstructivedisease | 6.0 (2.4, 15.1) | 0.7 (0.6, 1.0) |
| Methacholine wheezes, detecting asthma | 6.0 (1.5, 24.3) | 0.6 (0.4, 0.9) |
| Diminished breath sounds, detecting asthma | 4.2 (1.9, 9.5) | 0.3 (0.1, 0.6) |
(Adapted from McGee S: Evidence-Based Physical Diagnosis. Philadelphia, WB Saunders, 2001.)
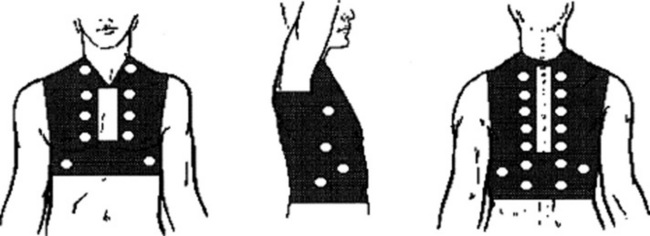
42 How can one objectively measure breath sounds’ intensity at the bedside?
By a scoring system originally devised by Pardee:
 Ask the patient to sit up and inspire from residual volume, fast and deep, while at the same time breathing through the mouth. This generates a breath sound as loud as possible.
Ask the patient to sit up and inspire from residual volume, fast and deep, while at the same time breathing through the mouth. This generates a breath sound as loud as possible.
 Auscultate bilaterally over the upper anterior zones, the mid-axillae, and the posterior bases.
Auscultate bilaterally over the upper anterior zones, the mid-axillae, and the posterior bases.
 Quantify the intensity of the inspiratory component of the vesicular breath sounds as: 0, absent; 1, barely audible; 2, faint but definitely audible; 3, normal; 4, louder than normal.
Quantify the intensity of the inspiratory component of the vesicular breath sounds as: 0, absent; 1, barely audible; 2, faint but definitely audible; 3, normal; 4, louder than normal.
 The sum of sound intensities recorded in each area generates the BSI score. This ranges from 0 to 24 (for, respectively, scores of 0 or 4 in each of the six areas).
The sum of sound intensities recorded in each area generates the BSI score. This ranges from 0 to 24 (for, respectively, scores of 0 or 4 in each of the six areas).
 To best use this method, you must disregard superimposed adventitious sounds (rhonchi, wheezes, or crackles), which, since they are louder than the underlying breath sound, would overestimate the BSI.
To best use this method, you must disregard superimposed adventitious sounds (rhonchi, wheezes, or crackles), which, since they are louder than the underlying breath sound, would overestimate the BSI.
44 In addition to airflow obstruction, is there any other process associated with distant breath sounds?
Pneumonia. A reduced BSI with fever and cough is very suggestive of it.
52 What is the cause of this improved transmission?
1. Consolidation (i.e., replacement of alveolar air with a mantle of solidified lung that can better transmit higher frequencies). Consolidation reflects either alveolar collapse or alveolar fluid-filling:
 Alveolar collapse (with patent airways) occurs in pleural effusions, whenever the amount of fluid is large enough to compress the alveoli but too small to compress the airways.
Alveolar collapse (with patent airways) occurs in pleural effusions, whenever the amount of fluid is large enough to compress the alveoli but too small to compress the airways. Alveolar fluid-filling occurs instead in situations of pneumonia (pus in the alveoli), alveolar hemorrhage (blood in the alveoli), or pulmonary edema (serum in the alveoli). In fact, in patients with cough and fever, the presence of bronchial breath sounds argues strongly in favor of pneumonia. Yet, its absence cannot rule it out, since the finding is specific but poorly sensitive.
Alveolar fluid-filling occurs instead in situations of pneumonia (pus in the alveoli), alveolar hemorrhage (blood in the alveoli), or pulmonary edema (serum in the alveoli). In fact, in patients with cough and fever, the presence of bronchial breath sounds argues strongly in favor of pneumonia. Yet, its absence cannot rule it out, since the finding is specific but poorly sensitive.2. Bronchial breath sounds also may be heard in situations of pulmonary fibrosis. This mechanism, however, requires severe fibrosis and tends to be less common than simple consolidation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree