It can’t be done.
It probably can be done, but it’s not worth doing.
I knew it was a good idea all along!
Arthur C. Clarke
- Although there have been advances in the tests available to diagnosis cancer, the initial history remains the crucial first “investigation.”
- Improvements in genetic tests and immunohistocytochemistry have ensured that the diagnosis can be made reliably and on increasingly small biopsy specimens. These tests also provide prognostic information about the diagnosis as well as predictive information, for example how the tumor will respond to specific biologically targeted therapies.
- Too many patients are still presenting with advanced disease, and effective screening programs exist for only a limited number of tumor types.
- Functional imaging in cancer with positron emission tomography (PET) and computed tomography (CT) is now standard of care for the main tumor types and provides superior information in discriminating malignant from benign disease. This ensures that patients are appropriately staged so that they are offered the best treatment available for them.
Introduction
In this chapter, presentations of some of the most common cancers are discussed. The range of diagnostic tests is reviewed, and the advantages and disadvantages of individual techniques are summarized. Future developments and potential new diagnostic techniques are reviewed (Box 17.1).
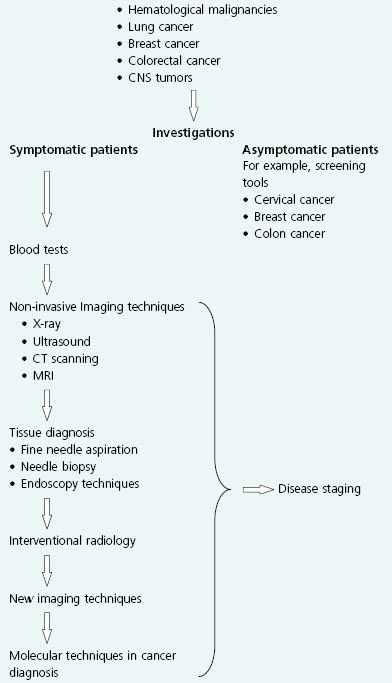
The diagnosis of cancer can be remarkably difficult to make. This is because patients may have nonspecific symptoms that mirror those found in common benign conditions. For example, a patient with colon cancer may have similar symptoms to a patient with the benign irritable bowel syndrome. In addition, some patients may hide their symptoms as a result of fear of the possible diagnosis of cancer and the likely treatment. Sadly, this means that many patients present late and already have advanced disease at the time of diagnosis.
It is essential to confirm that there is an underlying malignancy which is the cause of a patient’s symptoms, and this ultimately relies on obtaining a representative sample of tissue in most instances. This will also provide information on the type of tumor so that the appropriate treatment can be chosen. Unfortunately it is frequently difficult to establish the histological diagnosis to confirm a suspected cancer. Tumors often contain areas which vary markedly in their concentration of malignant cells and frequently have large islands of necrotic and acellular tissue. As a result, the site from which a biopsy is taken may not actually contain tumor cells. A classic example of this arises when a clinician tries to make a diagnosis of pancreatic cancer. A lesion in the head of the pancreas can cause a desmoplastic reaction (an inflammatory local process), which, when biopsied, reveals reactive rather than neoplastic cells. Further attempts at biopsy may fail, and the clinician is left with the difficult decision of whether to make the diagnosis on clinical grounds alone, with all the implications this has for the prognosis that is given to the family and the potential for toxicity of the treatment that will be offered.
Many of the investigative procedures used in oncology are uncomfortable and inconvenient for the patient. They are often difficult to interpret, with false positives and negatives. It is also important not to submit patients to unnecessary tests, which can cause morbidity (even mortality), huge costs to the healthcare system, and inappropriate distress. There should always be a logical sequence of investigations, and for many conditions there are now protocols to follow which should facilitate the ability to make an accurate diagnosis in the shortest time possible.
Thankfully, in oncology there has been a steady development of investigative procedures which are more reliable and less invasive. In the future, the hope is not only that diagnoses will be made swiftly with minimal discomfort to the patient, but also that more molecular information about the tumor can be obtained with the techniques available. By knowing the molecular signature of a tumor, it will be possible to individualize treatment using the increasing array of targeted therapies (see Chapter 16). This approach will increase the efficacy of anticancer therapy and also possibly reduce toxicity.
Clinical Manifestations
Patients with cancer present in a variety of ways depending on the local effect of the primary tumor or the effects of any sites of metastatic spread, or from an indirect metabolic effect such as hypercalcemia. There may also be nonspecific effects which are frequently seen with many tumor types and are believed to be due to production of a variety of cytokines by the tumor cells. These symptoms include anorexia, weight loss, and lethargy. Finally, a variety of malignancies may produce symptoms and signs distant from the primary site or its metastases – termed “paraneoplastic syndromes” – and these are often related to the central nervous system (CNS). The clinical manifestations of the hematological malignancies (lymphoma and leukemia) and solid tumors involving lung, breast, bowel, and nervous system are discussed in more detail in this chapter. Further information on presenting symptoms in other tumors can be found in general texts (e.g. DeVita et al., 2011).
Lymphoma and Leukemia
The vast majority of patients with lymphoma will present with nodal disease. The patient may notice painless enlargement of a lymph node, most commonly in the neck. Sometimes the nodes will fluctuate in size, and therefore the patient may not consider this to be serious. For patients with Hodgkin disease, the initial site of presentation is most often in the cervical nodes (70% of all cases), axilla (25%), and inguinal area (5%). In non-Hodgkin lymphomas, the nodal disease tends to be more widespread. Occasionally the lymph nodes will grow very rapidly and patients may notice symptoms due to resultant compression of blood vessels. This may cause swelling of a leg when there is extensive inguinal lymphadenopathy or superior vena cava obstruction due to mediastinal nodes. In non-Hodgkin lymphomas, enlargement of the retroperitoneal lymph nodes can cause backache and even renal failure due to obstruction of the ureters. Patients may present with obstructive jaundice if the nodes are enlarged in the porta hepatis and, in the more extensive lymphomas, the liver and spleen may be involved causing their enlargement with associated abdominal discomfort. Lymphomas often cause constitutional symptoms which may be the presenting features. Fever is the most frequent of these and may occur with drenching night sweats. Weight loss is common and is usually more marked in those patients with bulky disease. The triad of constitutional symptoms (fever, sweats, and more than 10% loss in body weight) are known as B symptoms, and the presence of these is associated with more advanced disease and poorer prognosis. Some patients with Hodgkin disease develop pain in the enlarged lymph nodes on drinking alcohol.
In leukemias, the most common presenting symptoms are fever, infection, malaise, and bleeding. These occur due to neutropenia, anemia, and thrombocytopenia, which are secondary to bone marrow infiltration by leukemic blasts. Some patients will present with bone or joint pain, and others may notice lymphadenopathy or develop abdominal pain from hepatosplenomegaly.
Lung Cancer
Most patients with lung cancer present with symptoms which are directly related to the tumor. These include hemoptysis, cough, dyspnea, and chest pain. These are common symptoms from cigarette smoking as it is often associated with background lung disease, and they may therefore be ignored by the patient and clinician, delaying the diagnosis of lung cancer. Direct tumor invasion into the mediastinum can cause severe pain which may be misinterpreted as being of cardiac origin. If the tumor invades the left recurrent laryngeal nerve, the patient will develop hoarseness of the voice, which may be the only presenting symptom. Classic pneumonias that fail to clear despite adequate antibiotics should always be considered as suspicious of an underlying malignant obstructing lesion.
A Pancoast tumor is situated at the apex of the lung and may cause severe pain in the shoulder, chest wall, and arm. This is due to the direct invasion of the brachial plexus. If the tumor is found in the right main or upper lobe bronchus, compression of the great veins of the neck may cause superior vena cava obstruction. Clinically a patient will have swelling of the face, neck, and upper arms with plethora or cyanosis and dilated veins over the upper part of the chest wall. Some patients may present with a lump in the neck where the tumor has metastasized to lymph nodes, or they may develop symptoms from other metastatic sites such as bone pain or headache from bone or brain metastases respectively. Finally, there may be weight loss, nausea, and jaundice from metastases to the liver. It is extremely common for patients with lung cancer to also have constitutional symptoms such as anorexia, weight loss, weakness, and fatigue.
Breast Cancer
In breast cancer, the local effects of the disease may cause a lump in the breast, changes in the nipple, pain in the breast, or puckering of the skin overlying a lump. Some patients may notice a mass in the axilla, and if this is significant there may also be swelling of the affected arm. If the patient has an inflammatory breast cancer, the whole breast may be inflamed with firmness of the tissue and widespread erythema. Breast cancers metastasize most commonly to the bones, lungs, and liver. Therefore, the patient may present with symptoms from a secondary deposit, such as pain in the back due to a vertebral metastasis or upper abdominal discomfort from hepatomegally.
Colorectal Cancer
The symptoms of colorectal cancer vary with the site of the tumor. When this arises in the rectum, the majority of patients will notice a change in bowel habit associated with rectal bleeding. Sometimes the patient may report the feeling of incomplete evacuation of the bowels, tenesmus, which is an extremely distressing symptom. Lesions affecting the left side of the colon often cause a change in bowel habit and also sometimes bleeding. If the tumor actually obstructs the bowel, patients will present with colicky abdominal pain, vomiting, and ultimately absolute constipation. Those lesions affecting the cecum and the right side of the colon most commonly present with ill-defined abdominal pain and anemia due to occult blood loss from the tumor. As with other solid tumors, patients may also present with constitutional symptoms or symptoms relating to sites of metastatic spread.
Tumors Arising from the Nervous System
Tumors of the nervous system can be divided into those which arise centrally and those in the periphery. Patients with brain tumors may present with symptoms of raised intracranial pressure such as headache, which is often worse in the morning; drowsiness; and nausea and vomiting. The actual site of the tumor may cause specific symptoms. For example, a tumor in the motor cortex can cause paralysis, tumors in the temporal region are often associated with epilepsy, and tumors in the cerebellum typically cause ataxia, nystagmus, and double vision. There may be endocrine effects of central nervous tumors. These usually result when they arise in the pituitary, which can cause elevated hormone levels with resultant manifestations such as Cushing’s syndrome. These tumors may subsequently extend up and involve the cranial nerve pathways causing visual field defects. Sometimes the tumors may bleed and the patient will notice a sudden deterioration in vision associated with headache and signs of hypo-pituitarism.
Tumors involving the spinal cord cause symptoms depending on the site at which they arise. Patients may experience pain at that level and, if there is resultant compression of the spinal cord, weakness may occur below the lesion, with associated constipation, bladder dysfunction, and changes in sensation. This is a medical emergency requiring rapid intervention to reduce the risk of permanent paralysis.
Investigations in Oncological Practice
Interpretation of Hematological and Biochemical Tests
In general, routine blood tests do not make the diagnosis of cancer but may indicate that there is a serious underlying illness. The most common changes which can accompany a malignancy are anemia, polycythemia, neutropenia, leucocytosis, thrombocytosis or thrombocytopenia, elevated liver enzymes, and reduced renal function. The erythrocyte sedimentation rate may rise in patients with cancer, but it is a nonspecific test and is also elevated in infections and several other conditions. In the leukemias, the peripheral blood film is essential as there may be a normocytic normochromic anemia, and occasionally leukemic blast cells are present. There may be an excess of white cells and a low platelet count. If the tumor has spread to the bone marrow in solid malignancies, a leukoerythroblastic picture will be seen with circulating primitive cells. In some solid tumors, the routine full blood count will show normocytic, normochromic anemia and, in those tumors where there has been blood loss, an iron-deficient anemia occurs.
Routine biochemistry may indicate varying degrees of renal failure if the tumor has obstructed the renal outlet. If the tumor has blocked the biliary tract, an obstructive picture in the liver enzymes will be seen with an elevated bilirubin and alkaline phosphatase. Bone metastases and some lung cancers will also cause a rise in alkaline phosphatase. The liver transaminases, aspartate and alanine amino transferase, will be increased in patients with liver injury which may be from alcohol, infection, or cancer. Lactic dehydrogenase (LDH) is an enzyme associated with tissue injury and is often increased in patients with a large tumor burden, lymphoma, or liver metastases. An elevated corrected calcium level indicates hypercalcemia, which may be due to bone metastases, or is a metabolic consequence of an underlying tumor such as lymphoma. Low sodium (hyponatremia) may represent the syndrome of inappropriate ADH secretion, which is often associated with an underlying small-cell lung cancer. A low albumin may indicate liver involvement with tumor or be a general indicator of the poor nutritional state of the patient (Table 17.1). For further biochemical effects of tumors, see Pannall et al. (1997).
Table 17.1 Interpretation of blood results in the diagnosis of cancer
| Abnormal blood test | Possible interpretation |
| Low hemoglobin | Anemia due to bleeding from the tumor Anemia due to bone marrow involvement by the tumor |
| Elevated white cell count | Infection Bone marrow involvement by tumor |
| Low white cell count | Neutropenia due to chemotherapy Bone marrow failure |
| Elevated urea and creatinine | Renal impairment; may be due to the kidneys being obstructed by the tumor, dehydration, or damage by chemotherapy |
| Elevated urea with anemia | Gastrointestinal hemorrhage |
| Hyponatremia and hyperkalemia | Addison’s syndrome due to tumor involving the adrenal glands |
| Hyponatremia | Syndrome of inappropriate ADH secretion in small-cell lung cancer |
| Hypercalcemia | Indirect effect of metastatic cancer or bone metastases |
| Hypokalemia or hypomagnesemia | Renal tubular damage due to chemotherapy or diarrhea |
| Elevated C-reactive protein | Nonspecific indicator of infection or inflammation |
| Hypoalbuminemia | Liver impairment, or malnutrition or cachexia |
| Elevated alkaline phosphatase | Bone metastases or liver metastases |
| Elevated alkaline phosphatase with elevated bilirubin | Biliary obstruction |
| Elevated hepatic transaminases (AST or ALT) | Liver damage which could be due to chemotherapy or metastases |
Tumor Markers
Some tumors produce proteins which can be detected in the blood and can be used to indicate the presence of a malignancy. The value of different tumor markers varies; they can be useful in diagnosis, predicting prognosis or response to treatment and aiding follow-up of a patient. Tumor markers can be broadly divided into three groups: oncofetal proteins, cancer-related antigens, and hormones (Table 17.2).
Table 17.2 Tumor markers and their uses
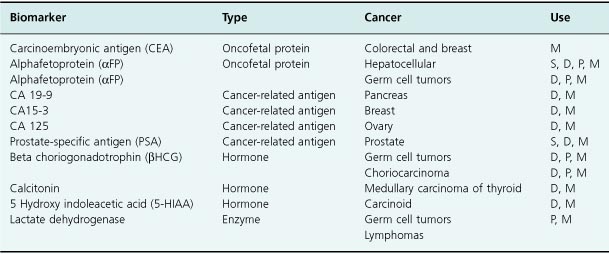
Note: D = diagnosis; P = prognosis; S = screening; M = monitoring of treatment.
An example of an oncofetal protein tumor marker is carcinoembryonic antigen (CEA). Although this marker may be produced in up to 80% of people with bowel cancer, it will also be elevated in patients with inflammatory bowel disease. Therefore CEA is usually not helpful in diagnosis but can be useful in measuring response to therapy or identifying the presence of recurrent disease at follow-up.
Cancer-related antigen tumor markers include prostate-specific antigen (PSA), CA125, and CA19-9. PSA is elevated in both inflammatory and malignant prostate disease. This means that it can support but not guarantee a diagnosis of cancer and can be very useful in monitoring the response to treatment. It has also been proposed as a marker to be used for population screening, but its utility as a screening tool remains controversial.
CA125 is elevated in approximately 80% of patients with ovarian cancer. Again, it can be extremely useful in monitoring response to treatment and detecting early relapse during follow-up. Indeed, in many patients the serum CA125 may become elevated before there is radiologically confirmed disease relapse, and as has been discussed at various points in other chapters in relation to prostate cancer, is this useful if the appropriate action is then uncertain? With this in mind, a randomized study has confirmed that early introduction of chemotherapy in ovarian cancer does not improve overall survival (Rustin et al., 2010).
CA19-9 is a tumor marker that is elevated in approximately 70% of patients with pancreatic malignancy. It is often a useful diagnostic tool, in combination with biopsy, to establish the etiology of pancreatic lesions. It can be particularly valuable to support a diagnosis in the significant number of patients for whom histological confirmation proves impossible.
Examples of hormones that are also tumor markers include human chorionic gonadotrophin (HCG) and calcitonin. An elevated HCG is pathognomonic of choriocarcinoma and in combination with alpha fetoprotein (AFP) is highly specific for testicular teratoma. These tumor markers are valuable as prognostic indicators in patients with teratoma, in addition to enabling the monitoring of response to treatment. Calcitonin is elevated in patients with medullary carcinoma of the thyroid and is used in detection, diagnosis, and follow-up after treatment.
There are now published recommendations for the use of tumor markers in clinical practice, for example through the American Society of Clinical Oncology. In the future, it is hoped that these markers will have greater specificity and sensitivity.
Genetic Tests
Diagnostic DNA testing is now available for a number of hereditary cancers. These include von Hippel–Lindau disease, breast and ovarian cancer syndrome, and the Li–Fraumeni syndrome. DNA is usually extracted from blood cells and profiled for cancer predisposition genes and mutations. When a specific gene mutation has been identified in other family members with cancer, a relative can be screened to determine whether they carry that specific mutation and whether this places them at high risk of developing the malignancy themselves. Genetic testing can therefore provide some individuals with their personal lifetime risk of developing a cancer. These presymptomatic subjects can then enter intensive screening programs, or even elect to have prophylactic surgery performed. An example of this would be the decision to undergo bilateral mastectomy for a woman whose family carries the mutated BRCA1 gene. (See Tables 17.3 and 17.4.)
Table 17.3 Chromosomal analysis in leukemia
| Leukemia | Abnormality | Implication |
| CML | t(9;22)(q34.1;q11.2) | Diagnostic |
| CLL | Trisomy 12 | Poor prognosis |
| Deletion 13q14 | Worst prognosis | |
| ALL | t(9;22)(q34.1;q11.2) | Poor prognosis |
| t(11;14(p13;q11) | Poor prognosis | |
| t(8;14)(q24;q11) | Poor prognosis | |
| AML M2 | t(8;21)(q22;q21) | Good prognosis in young adults |
| M3 | t(15;17)(q22;q11) | Best prognosis of all AMLs |
| M4 | Inversion of 16 | Good prognosis |
| M5 | 11q23 abnormalities | Poor prognosis |
Table 17.4 Familial cancer syndromes
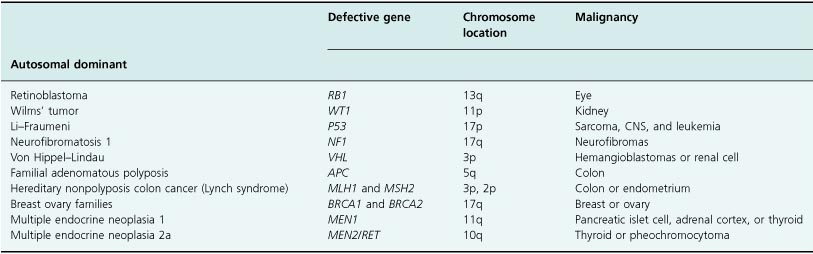
Inherited genetic predisposition might contribute to the development of many cancers (see Chapter 3), but in the vast majority of cases may prove too complex to provide a readily applicable screening tool in the foreseeable future. In addition, mutations can occur during life in genetic sites which have not yet been identified. This therefore means that while DNA testing is currently available, it is actually helpful in only a small number of people, representing a minority of patients with cancer. The sequencing of the human genome has focused huge amounts of research in this area, and in the future the value of genetic testing is likely to increase for the whole population.
Screening Tests
The objective of screening a population for cancer is to detect tumors at the earliest possible stage while they are potentially treatable and thus curable. This straightforward aim is actually a complex process. In an ideal screening program, the natural history of the disease should be well understood with a recognizable early stage that is treatable. Both the sensitivity (the chance that someone with the disease will test positive) and the specificity (the chance that someone without the condition will test negative) should be high. The test should be acceptable to the public, and the cost should be balanced against the benefit it provides. Unfortunately most proposed screening tests have failed to meet all of these criteria. Interpretation of the evidence is made difficult by two key biases in the data, firstly the so-called lead time bias. This occurs when a cancer is detected earlier than would be normally expected, but due to ineffective treatment the date of death remains the same. Screening in this case has produced no benefit, but the time from diagnosis to death has increased, giving an illusion of benefit. The second bias is related to a tendency to pick up more benign, slow-growing disease in screening tests. This has two consequences, firstly causing an average improvement in prognosis for the whole group and thus giving a false impression of the effect of screening, and secondly potentially overtreating the population with benign disease. This, along with the extra anxiety that a positive test causes (for every breast cancer detected at screening, 10 women are recalled for assessment), makes the analysis of cost-effectiveness for all screening programs a difficult one.
Cervical Cytology
The screening test used in the early detection of cervical cancer is the Papanicolaou smear (Pap smear). This involves taking a scraping of cells from the transformation zone of the cervix (the area where columnar epithelium changes to squamous morphology). The precancerous lesion in the cervix is cervical intraepithelial neoplasia (CIN). Cytological studies can grade the CIN from I to III where CIN III is the most severe grade (approximately 30% of these precancerous lesions will develop into invasive carcinomas if left untreated). By treating patients with CIN changes, it is thought that the incidence of invasive cancers could be reduced by up to 90% if the screening test was offered to women every 3 years. There has also been interest in screening patients for the human papillomavirus (HPV), which is present in the majority of women with cervical malignancies. In the United Kingdom, a vaccination program has been in use since 2008 to vaccinate girls aged under 18 years against HPV 16 and 18 in an attempt to prevent them getting cervical cancer.
In practice there have been problems with the cervical cytology-screening program. Some of these difficulties have been related to the accurate reporting of CIN I to III. Quality assurance and audit have, to a large extent, rectified the interobserver variability that was a concern in early years. However, those most at risk of cervical cancer are women who have been sexually active with multiple partners at a young age, and who are generally of poor socioeconomic background. These are not typically the individuals who come forward for screening, and efforts are still ongoing to encourage these women to attend.
Mammography
Mammography involves an X-ray examination of the breast. There have been numerous randomized controlled studies investigating the effectiveness of mammography to detect breast cancers at an early stage. At the outset, the use of mammography as a screening test for breast cancer held much promise as this is one of the commonest malignancies, and mammography can detect tumors before patients are themselves aware of them. However, to date the overall results from screening programs worldwide have been the subject of much debate with doubt being raised over whether real reductions in mortality have been seen. There does seem to be an increased rate in mammographic detection of early cancer, but this does not necessarily correlate with a survival advantage. The false positive rate is considerable which makes the cost of screening high, both financially and in terms of the anxiety to women. A Danish study suggested no change in death rates in two populations with and without screening in Denmark, and the following correspondence rebutting this paper gives a feel for the controversy in this area. Whatever your individual conclusion as to the worth of breast-screening programs, it is clear that the benefit to the individual is not guaranteed, and there is a risk of overtreatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


