- The incidence of cancer in developed countries is rising due to increased aging of the population and increasing exposure to carcinogens.
- Cancers can cause symptoms from local mass effects, leading to pain or organ dysfunction, or may cause systemic symptoms. These latter may be nonspecific, including weight loss, lethargy, muscle wasting, and debility, or predictable arising from effects of secreted proteins.
- A suspected diagnosis of cancer should be confirmed by histological assessment of biopsy specimens taken from areas of abnormal tissue and may be supported by results of imaging and serum markers.
- Lung cancer is the commonest malignancy causing death in Western nations and comprises small-cell and non-small-cell variants, which each have different biological behaviors, treatments, and outcomes.
- Breast cancer is the commonest malignancy in women and has widely differing incidences in different nations, suggesting the importance of environmental factors in its etiology.
- Prostate cancer has a 10-fold difference in incidence between Japanese and Afro-Caribbean men and carries a worse prognosis in the Afro-Caribbean population.
Introduction
One death is a tragedy, 100 000 deaths are statistics.
Albert Szent-Gyorgyi
It is estimated that worldwide there are approximately 10 million new cases of cancer per year, causing 6 million deaths. By 2012, with a worldwide population estimated at 8 billion, it is anticipated that at least 20 million new cancer patients will be diagnosed each year, causing 12 million deaths. Currently, around half of all cancers occur in developing nations, a figure that is expected to increase to 70% over the coming decade. However, this still represents a much smaller percentage of the overall population when compared to developed countries; incidence is approximately 100 per 100 000 population whereas it is 3- to 4-fold higher in Western nations. In fact if non-melanoma skin cancers are included, then around 1 in 3 of us can expect to develop some form of malignancy during our lifetime. Cancer is the number three killer in developing countries, behind infection and cardiovascular diseases, but has claimed the number two spot in developed countries after cardiovascular disease. In fact, if we consider the comparative ease with which we could forestall risk of cardiovascular diseases and with an ever aging population one would not bet against cancer reaching the gold medal position before the end of the next century.
In 2004, based on World Health Organization (WHO) reports, 7.4 million people died as a result of cancer worldwide. The commonest type of cancer was lung, which accounted for 1.3 million deaths and then stomach (803 000 deaths), colorectal (639 000 deaths), liver (610 000 deaths), and breast (519 000 deaths). Figures for men revealed that the most frequest types of cancer worldwide (in order of the number of global deaths) were lung, stomach, liver, colorectal, esophagus, and prostate, whereas for women the order of frequency was breast, lung, stomach, colorectal, and cervical cancers. Over the last 20 years there has been a global increase of malignancies ascribed to HIV/AIDS, with marked increases of lymphomas and hepatocellular carcinomas.
The incidence of cancer in Western societies has risen steadily over the last century, predominantly because of the increasing median age of the population but also because of rising exposure to carcinogens. In fact, in Europe and North America cigarette smoking and dietary factors are major or contributory factors to the development of up to two-thirds of all malignancies. Lung cancer is by far the commonest cause of death from malignancy in both males and females, followed by colorectal cancer in men and breast cancer in women.
This chapter will focus on the clinical features of the most important cancers and will include information on clinical manifestations, diagnosis and treatment (Box 2.1). More detailed information on diagnostic procedures is given in Chapter 17.
- Lung
- Breast
- Colorectal
- Prostate
- Renal
- Skin
- Cervix
- Hematological
- Lymphomas
- Leukemias
- Lymphomas
- Pathology
- Etiology
- Local symptoms from primary
- Symptoms from metastases
- Systemic symptoms of malignancy
- Paraneoplastic syndromes
- Symptoms from biochemical abnormalities
- Surgery
- Chemotherapy
- Adjuvant
- Neoadjuvant
- Advanced
- Adjuvant
- Hormones
- Radiotherapy
- Biological therapies
- Modulators of cell signaling pathways
Any malignancy may cause symptoms, resulting from various biological effects on the host. The earliest manifestations may be caused by the local effect of the tumor, including the presence of a mass, discomfort from compression of local organs or nerves, hemorrhage from the involvement of blood vessels, or obstruction of airways, ureter, bile duct, and other key structures. Tumors may also present with nonspecific effects such as cachexia (the loss of body mass frequently seen in patients with chronic debilitating diseases), lethargy, weight loss, fever, and a variety of neuromuscular syndromes. Although more usually associated with benign hyperplasia or adenomas arising in tissues that are normally hormone-secreting (endocrine tissues), some malignancies may produce hormones acting on distant tissues via the circulation. Such hormones include ADH, PTH, erythropoeitin, ACTH, calcitonin, and even members of the IGF family of growth factors, such as IGF-II, which can all cause distant effects that may prove life-threatening. More common, however, is the near ubiquitous production of growth factors whose action is confined to the tumor, blood vessels, and surrounding stroma. Ultimately, most cancer deaths are the result of invasion and spread of the primary cancer to other more distant tissues (metastases), which may in some cases become clinically apparent before the primary tumor. Indeed, in some cases the primary may never be discovered. The diagnosis of malignancy can therefore be difficult with a wide variety of presentations, which may not necessarily indicate an obvious site of the primary tumor.
For those patients who complain of specific symptoms that raise the possibility of a particular malignancy (e.g. altered bowel habit and carcinoma of the colon), the necessary investigations and diagnostic procedures are usually self-evident. For those patients who present with nonspecific symptoms (e.g. cachexia and weight loss) the choice of investigations is difficult. The majority of such individuals will probably not have an underlying malignancy and it is essential to balance the potential morbidity and occasional mortality of investigations (together with the cost) against the likelihood of detecting a malignancy and the potential value of making an early diagnosis. The “gold standard” of diagnosis is obtaining tissue that, on histological examination, confirms the presence of neoplastic cells. In the majority of cases this is straightforward, with symptoms guiding the choice of a site for biopsies. Support for a diagnosis can be provided by tumor markers (usually proteins specifically expressed by or in response to a tumor) and abnormalities of hematological and biochemical blood results, but in a small percentage of patients no obvious site of neoplastic focus is detected and a diagnosis has to be made on the basis of probabilities.
A further occasional clinical problem arises when a metastatic site is detected (most commonly an isolated area of lymphadenopathy) and histological examination reveals malignant cells (frequently adenocarcinoma or squamous carcinoma). Investigation of common primary sites (e.g. breast, gastrointestinal tract, head and neck, lung) reveals no obvious origin and in this instance a diagnosis of “carcinoma of unknown primary” is made, which can lead to a dilemma when trying to choose appropriate therapies. Some of the reasons why cancer cells may diverge so far from their cell type of origin so as to become unrecognizable are discussed in the following chapters.
Key features, particularly the prevalence, pathogenesis, and clinical manifestations of selected cancers, are outlined below. Current approaches to diagnostic techniques and treatment are summarized. It must be stressed that approaches to diagnosis and management of cancer are changing rapidly and many new methods of diagnosis, including new imaging techniques and markers, are constantly becoming available. In addition, numerous new targeted therapies that are not chemotherapy drugs have been included in treatment regimens. The following chapters will give a fair idea of what processes and molecules we predict to become the next generation of cancer therapeutic targets, whereas here we concentrate on what is already part of clinical practice.
Lung Cancer
Smoking is hateful to the nose, harmful to the brain, and dangerous to the lungs.
King James I
Incidence
The incidence of carcinoma of the bronchus rose steadily throughout the twentieth century in the Western world, closely mirroring the increase in cigarette smoking. Of all malignancies, lung cancer has the highest mortality rates, with the death rate approaching that of incidence. As a result in the Western world, it has long been the main cause of cancer-related death in men, and has become the main cause in women since the late 1980s. The overall annual incidence in most Western nations is approximately 100 per 100 000 males and 40 per 100 000 females. There has been a decline in the incidence among males since the late 1990s, but as smoking has become more popular in females, there has been an increasing incidence in women, such that the male : female ratio for lung cancer incidence has risen steadily from approximately 13 : 1 in the 1950s to 2 : 1. The global rise in lung cancer incidence following a marked increase in smoking in the Middle East, Asia, and Africa is of concern. It was estimated that during 2005 approximately 500 000 new cases of lung cancer were diagnosed in China.
Between 80% and 90% of all lung cancers are attributable to cigarette smoking. The first study to clearly demonstrate this association was published by Doll and Hill in 1964 and was based on the incidence of lung cancer among doctors in the United Kingdom (Box 2.2). Several subsequent studies have confirmed this link and have also shown a relationship between the number of cigarettes smoked and the risk of lung cancer. Importantly, there is also clear evidence that the cessation of smoking is associated with a subsequent fall in lung cancer risk such that approximately 12 years after discontinuation, the risk of lung cancer almost reaches that of a life-long nonsmoker. There has also been an observation that lung cancer risk rises among nonsmokers who live or work in an environment with smokers, leading to an approximately twofold rise in the risk of lung cancer (“passive smoking”). Young females who smoke appear to be at a particularly high risk of subsequent lung cancer, an extremely worrying observation considering the current vogue for cigarette smoking among young peri-pubertal females in most Western nations.
Other factors that might increase the risk of lung cancer include exposure to asbestos, radon, industrial air pollution, chromium, nickel, and inorganic arsenic compounds. Of these, asbestos exposure appears to have the greatest impact for both lung cancer and for mesothelioma (a cancer of the lung lining that is very rare in those not exposed to asbestos). The effects of carcinogens are additive, as exemplified by the risk of lung cancer in an asbestos worker who also smokes, which is a staggering 45-fold that of the normal population.
As a recent example of the potential value of identifying genetic susceptibility for cancer (see Chapter 3), there is now good supporting evidence that polymorphic alleles for genes encoding phase I/II metabolizing enzymes may influence the production and clearance of carcinogens and could be identified as biomarkers for individuals particularly vulnerable to the carcinogenic effects of inhaling tobacco smoke. Numerous studies have investigated the possible link between other genetic abnormalities and the risk of developing lung cancer (discussed in detail in Chapter 3). Thus, for instance, activating mutations in KRAS, upregulation of the epidermal growth factor receptor (EGFR), and inactivating mutations in tumor suppressor signaling, particularly p53, are ubiquitous in non-small-cell lung cancers (NSCLCs), but in most cases result from somatic mutations. Therefore, such knowledge may have limited value in developing biomarkers for early diagnosis but might instead increasingly in coming years be employed to determine prognosis and to guide subsequent chemotherapy. To date, although numerous genetic changes have been reported, no clear links between specific hereditary genetic abnormalities and the risk of most lung cancer have been proven.
Pathology
Lung cancers are divided into two major subgroups – small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC); these differ markedly in respects to their biology, therapeutic sensitivity, and outcomes. NSCLC is further separated, on the basis of pathological features, into subgroups, including squamous cell and adenocarcinoma. For reasons that remain unclear – but do not appear to include smoking behavior – the incidence of adenocarcinoma, which currently accounts for 40% of all lung cancers, is continuing to increase. Adenocarcinomas frequently originate at the lung periphery and will often invade the pleura. Squamous cell carcinoma was the most frequent subtype until the early 1990s, since when its incidence has been reducing. Squamous cell carcinomas are more likely to be centrally placed and may be more easily detected by cytology. As these tumors grow, they may obstruct major airways with resulting distal pneumonia. They appear to begin as in situ carcinomas with subsequent development over 3–5 years before they become clinically apparent. A third group of less differentiated large cell carcinomas comprise only some 15% of all NSCLCs and usually arise in more distal bronchi.
Small-cell lung cancer is identified by the presence of diffuse small cells, which contain fine granular nuclei. Granules are frequently seen and contain a variety of hormones, including ACTH and ADH. Markers of neural differentiation are often expressed by these tumors and overexpression of one or more of the oncogenes from the MYC family is frequently observed (see Chapter 6). Between 20% and 25% of all lung cancers show a mixed picture, frequently with a small and non-small-cell component. The cellular origin of lung malignancies remains controversial and this is discussed later in the book under the umbrella of the cancer stem cell debate.
Clinical Features
Although lung cancer is the most common cause of paraneoplastic syndromes, most patients present with symptoms related to the primary tumor. Initially there may be a cough, which is often persistent and may be associated with a wheeze. This is frequently confused with similar symptoms induced by smoking and may simply be perceived as an exacerbation of existing chronic obstructive airways disease. Consequently, patients often delay seeking medical advice. Hemoptysis may follow, with frequent blood-streaked sputum but rarely massive hemorrhage. Dyspnea may result from obstruction of an airway or the development of a pleural effusion. This may be exacerbated by segmental collapse of one of the lungs. Pain is a frequent associated feature and may indicate mediastinal involvement or invasion of ribs or pleura.
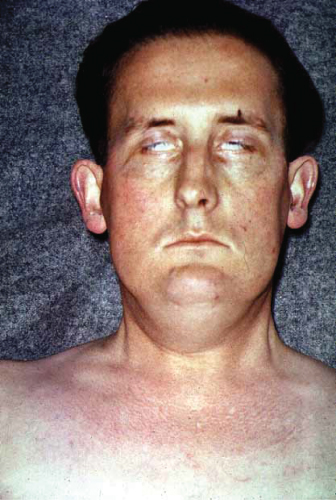
Hoarseness of the voice is seen when tumor invasion of the recurrent laryngeal nerve occurs and dysphagia may occur with mediastinal lymphadenopathy (enlarged lymph nodes in the chest), which compresses the esophagus. Apical tumors (arising in the uppermost area of lung) may compress the superior vena cava, causing the syndrome of superior vena cava obstruction (SVCO). This is associated with a reduced return of venous blood from the head and neck, resulting in swelling of the face, arms, and neck (Fig. 2.1).
Figure 2.1 Superior vena cava occlusion leads to marked swelling of neck, arms, and dilation of vessels on the chest wall. In this patient it resulted from large apical carcinoma of the lung.
Numerous symptoms follow the development of metastases, and may relate directly to the site of the secondary tumors, including bone pain, liver capsule distension from hepatomegaly, headaches, and epileptic fits from cerebral metastases (Fig. 2.2) and discomfort at the sites of lymphadenopathy. Some patients may actually present with a mass in lymph nodes (commonly in the neck) from a metastasis.
Figure 2.2 Bilateral ptosis caused by cerebral metastases from small-cell lung cancer. This patient had metastases involving the cerebral hemispheres and brainstem.
Several paraneoplastic syndromes are associated with lung cancer, predominantly SCLC. Some of these may result from the abnormal production and release of hormones by the SCLC:
- The syndrome of inappropriate ADH secretion (SIADH) is associated with profound hyponatremia (due to water retention, secondary to the action of ADH on the kidney), causing lethargy and somnolence.
- The production of a protein related to parathyroid hormone, PTHrp, may cause hypercalcemia, leading to nausea, polyuria, and, if untreated, coma.
- The elevated and unregulated secretion of ACTH (“ectopic ACTH” – because it is not coming from its usual source in the pituitary gland) may result in dramatic increases in secretion of the steroid hormone cortisol from the adrenal gland, leading to Cushing’s syndrome.
In fact, ectopic production of protein hormones by cancer cells is often far more problematic than the excess production of the same or related hormones from their usual cell type. This is primarily because mutations or altered differentiation of the cancer cells that have conferred the ability to produce these hormones are rarely, if ever, matched by the production of proteins needed for feedback control – there are no brakes on this system. Other paraneoplastic effects can include cerebellar syndromes, muscle weakness (myasthenia), and a variety of other neurological abnormalities.
Small-cell lung cancer has a more aggressive rate of development than NSCLC and has one of the highest tumor doubling times of all solid tumors. As a result it is rarely localized at the time of diagnosis and, almost invariably, metastases can be detected. Bone marrow metastasis is frequent and can be identified in up to 90% of cases by using sensitive assays for detecting cancer cell genes such as polymerase chain reaction (PCR) or sequencing. Left untreated, SCLC results in rapidly progressing symptoms over as little as 2–3 months, whereas the rate of progression with NSCLC is much slower, with a proportion of patients having truly localized disease. This difference in the biological behavior of the disease has a significant impact on management.
Diagnostic and Staging Investigations
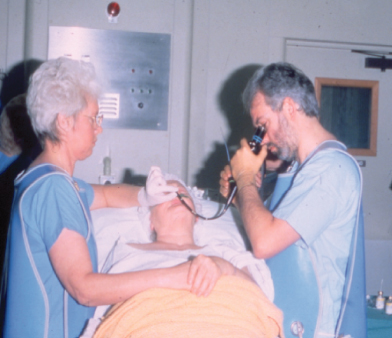
The possibility of lung cancer may first be raised when an opacity (“shadow”) is seen on a chest X-ray, which may be a mass often associated with an area of pneumonia. Confirmation of a diagnosis relies on obtaining neoplastic cells and in almost all cases this is undertaken by fiberoptic bronchoscopy, which is a rapid outpatient procedure allowing direct visualization and biopsy of the majority of tumors (Fig. 2.3). Brushings may also be taken from the bronchi and can reveal the presence of cancer cells (cytological evidence) in up to 80% of cases. In some cases metastases, usually lymph nodes, may be biopsied to confirm a suspicious X-ray.
Figure 2.3 During bronchoscopy the patient can be either lying down or sitting upright. The procedure is undertaken with local anaesthetic to the throat only in most cases.
Following diagnosis, staging is undertaken to determine the extent of disease. For SCLC, surgical resection is unlikely to be performed and staging is therefore relatively simple, the aim being to obtain a rough estimate of the total tumor burden and divide patients into those who will receive more or less aggressive therapy (see Chapter 18). Staging for SCLC is divided into “limited” disease, in which tumor is confined to one side of the chest hemithorax (a third of patients), or “extensive” disease, where cancer has spread beyond this (60–70% of patients). Staging involves blood tests for routine biochemistry, liver function, and full blood count, and often isotope bone scans and imaging of the liver either by ultrasound or computed tomography (CT) scanning. If blood counts are abnormal, it is practice in many centers to undertake a bone marrow examination. If there is any clinical evidence of cerebral metastases, imaging of the brain is undertaken.
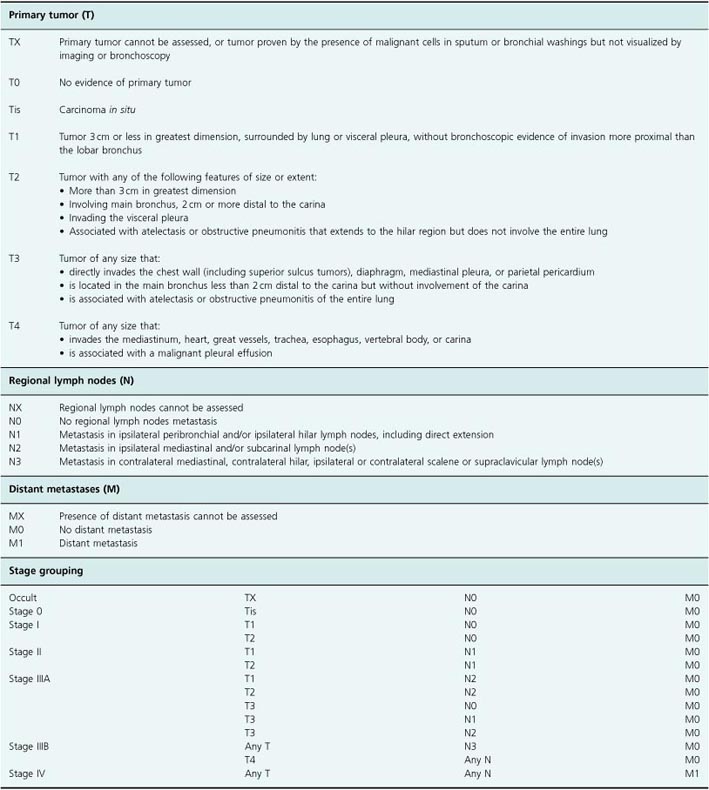
For NSCLC, staging is more important as surgical resection may be possible and it is essential to exclude metastatic disease for those who might undergo surgery. A widely accepted TNM (tumor, node, metastasis) staging system is used that divides patients into stage I (a), (b), stage II (a), (b), stage III (a), (b), or stage IV disease (Table 2.1). Survival is closely linked to stage. Staging investigations include rigid bronchoscopy in many centers for those patients considered potential candidates for resection, CT scanning of the thorax and abdomen, mediastinoscopy to directly visualize the mediastinum and, increasingly, positron emission tomography (PET) imaging. These investigations complement each other to minimize the risk of undertaking a futile major surgical procedure (thoracotomy) on a patient with metastatic disease.
Table 2.1 TNM staging of non-small-cell lung cancer
Treatment
Surgery
Surgical resection is the only form of therapy that provides the possibility of cure in NSCLC. This reflects the potential for this disease to be detected at a time prior to dissemination. Unfortunately, metastases are almost invariable present in SCLC patients (albeit microscopic) and so surgery is rarely offered. An exception, in some centers, is for patients with small peripheral lesions that have been detected coincidentally.
For all patients with NSCLC, the potential for resectability is considered in the first instance. Such surgery is only undertaken in patients with localized disease (stage I/II). Approximately 30% of patients will be offered resection (many will be excluded because the cancer is deemed inoperable or the patient’s general condition is poor). Surgery depends on the site of disease, and may include local segmental or wedge resections, lobectomy, or even pneumonectomy (removal of an entire lung). Success of surgery depends on the stage of cancer, with 5-year survival rates of approximately 54% in stage I, but only 24% for stage IIb. For those centers who operate on stage IIIa disease, neoadjuvant chemotherapy is now frequently offered and combined with radiotherapy. Several studies have shown this to significantly improve median survival up to 20–30% in some series. Postoperative adjuvant chemotherapy has been used increasingly since the mid-2000s after several large randomized studies demonstrated prolonged survival and reduced recurrence rates with cisplatin-based regimens in radically resected stage II and IIIa NSCLCs.
Radiotherapy
Radiotherapy is used to good effect in NSCLC to manage immediate symptoms or to delay the time until symptomatic progression rather than in an attempt to cure (palliation) (see also Chapters 18 and 19). SVCO also often responds to radiation treatment. The timing of palliative radiotherapy remains controversial, with no clear evidence that early initiation prior to the development of symptoms provides better quality of life or improved survival compared with waiting for symptoms to develop and treating at that point. Nevertheless, in most centers, early radiotherapy is initiated. Radical radiotherapy may occasionally be offered to patients with the intent of producing long-term survival. In particular, this option is favored for localized disease unsuitable for surgery, but the 5-year survival is only 10%. Continuous hyperfractionated accelerated radiotherapy (CHART) has been demonstrated to improve survival (by approximately 10%) compared with standard techniques. Improved survival has also been demonstrated with the addition of cisplatin to radiotherapy. Both approaches are now widely adopted.
Radiotherapy is frequently used in SCLC as this is one of the most radiosensitive of all tumors. Rapid reduction in tumor volume occurs in 80–90% of patients but recurrence is almost inevitable. The addition of radiotherapy to chemotherapy regimens appears to improve median and 2-year survival rates by approximately 5%. For patients with limited-stage disease, thoracic radiotherapy is widely used early during the administration of chemotherapy and prophylactic cranial radiation is also routinely offered with chemotherapy, reducing the frequency of brain metastases from approximately 40% of all patients to 8%. Local radiotherapy for the palliative treatment of painful metastases is of frequent value during the course of disease.
Chemotherapy
Chemotherapy is widely utilized in the treatment of all forms of lung cancer. A wide variety of combinations of agents are used in NSCLC as single-agent response rates are relatively low at 15–30%. The most commonly used agents are cisplatin, carboplatin, gemcitabine, paclitaxel, docetaxel, vinorelbine, topotecan, and ifosfamide (see Chapter 18). Many combinations have been compared and it appears that so long as a platinum agent is included, activity for two or more agents is similar with response rates of 40–60%. National guidelines suggest that standard of care should be with a platinum-containing doublet in patients who can tolerate platinum compounds.
Several targeted agents (see Chapter 16) have been examined in the management of NSCLC, and bevacizumab, the monoclonal antibody against vascular endothelial growth factor (VEGF), has produced significant survival benefits when combined with paclitaxel and carboplatin. It was found to cause increased rates of hemorrhage in squamous carcinomas and is therefore only approved for nonsquamous histology in patients without evidence of brain metastasis. As is the case for many solid and hematological malignancies, there is now increasing enthusiasm for the use of EGFR inhibitors (see Chapter 5). EGFR signaling is increased in 40–80% of lung cancers and two EGFR inhibitors – gefitinib and erlotinib – have been included in large randomized trials. Response rates of up to 20% have been seen with gefitinib in pretreated patients, but two large randomized trials of chemotherapy with or without gefitinib proved negative. Significantly better survival is seen in a subgroup of patients who have never smoked, are of Asian origin, have adenocarcinoma, and are female. Erlotinib has been shown to produce response rates of approximately 9% in patients who have been pretreated with chemotherapy.
Recent analysis suggests that mutations of the EGFR gene predict improved outcome and use of EGFR inhibitors is increasingly focused on this subgroup. There is also growing interest in many other targeted agents in NSCLC and this is in line with the increasingly prevalent philosophy of “tailored” or individualized medicine, where subgroups or even individuals are selected by molecular diagnostic techniques to identify those best suited to a given therapy.
For inoperable disease, the early introduction of chemotherapy appears to have significant, though modest, benefit in terms of median and 1-year survival and is associated with an improvement in the quality of life. Surprisingly, the early use of chemotherapy also appears to be cost-effective when compared with palliative care alone. The use of preoperative chemotherapy for those deemed surgically resectable is gaining increasing support. Large randomized trials comparing surgery alone with preoperative chemotherapy are ongoing as the results of published studies to date remain conflicting. One meta-analysis, however, has suggested an improvement of 1-year survival of approximately 5%.
Chemotherapy has a more established role in the management of SCLC. This disease is highly chemosensitive, with single-agent response rates of approximately 60% for carboplatin and etoposide and a slightly lower response rate of 30–40% for a wide variety of other agents. Limited-stage disease is usually treated aggressively with combinations of agents (including cisplatin or carboplatin for best results) and a median survival of 14–16 months is now achieved (compared with just 4 months without treatment). In fact, up to 10% may survive beyond 5 years. For those with extensive-stage disease, chemotherapy is offered for palliation and, as a result, regimens are chosen with less toxicity. Median survival rates of 8–10 months are usually achieved, with approximately 2% of patients alive at 2 years.
Breast Cancer
Breast cancer is the most common malignancy in women in Western nations and the second most frequent cause of cancer death (estimated to account for 26% of cancer cases and resulting in 15% of cancer deaths). The highest frequency is in the United States with an annual incidence of 85 per 100 000. In Europe, it is commonest in the Netherlands with an incidence of 70 per 100 000/year and lowest in Spain with an incidence of 43 per 100 000/year. It is much less common in Asia, and Japan has an incidence of just 25 per 100 000. The incidence increased steadily during the twentieth century but has plateaued or reduced since then. The death rate from breast cancer decreased by approximately 24% in the United States since 1990 and similar reductions have been seen in Europe. On average, approximately one in nine women develop breast cancer in Western nations, and one in three of these will die of their disease.
Many potential risk factors for the development of breast cancer are known. A family history of carcinoma of the breast increases the risk, with one first-degree relative conferring a threefold increase and an even greater risk if the relative was premenopausal when diagnosed. Lifetime exposure to estrogen is an important risk factor, with breast cancer risk increased by early menarche or late menopause and by use of hormone replacement therapy after menopause. Women who have their first child over age 30 years experience a threefold increased risk. Previous benign breast disease, a prior exposure to radiation and high dietary fat intake all appear to increase the risk of breast cancer. A factor which has recently been shown to be an important predictor of breast cancer risk is the detection of increased mammographic breast density seen on screening.
The key role of inherited genetic factors in breast cancer was illustrated by the identification of mutations in the BRCA genes in patients with familial breast and ovarian cancer (see Chapters 3 and 10). Mutations of this gene are associated with a 50–85% risk of developing breast cancer during the lifetime of a woman. Other inherited mutations are being identified.
Pathology
The majority of breast cancers are adenocarcinomas, which may be either infiltrating lobular or ductal. With the increasing uptake of mammographic screening, more preinvasive tumors – ductal or lobular carcinoma in situ – are being detected. Rarely, primary lymphomas, sarcomas, and squamous carcinomas may be found in the breast.
Clinical Features
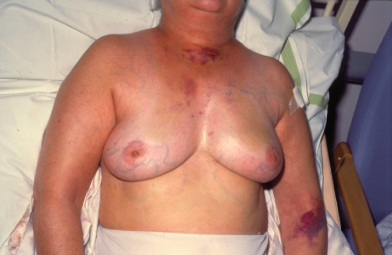
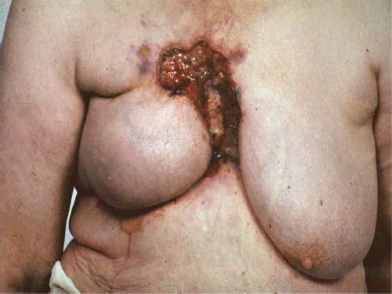
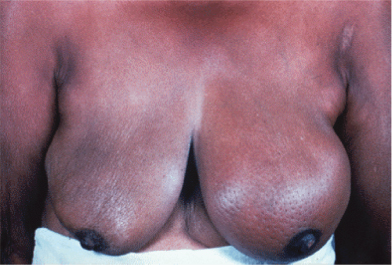
Breast cancers vary considerably between individuals in their rate of growth and pattern of spread. Some may remain predominantly localized, infiltrating local structures, others may spread via lymphatics to draining lymph node areas, while others may disseminate widely in the bloodstream. The majority of women will present with a lump in the breast and some will have pain in this region, discharge, or bleeding from the nipples. Increasing numbers of women are being diagnosed following screening and will have no symptoms (Fig. 2.4). In some cases, local infiltration can produce a fungating tumor with erosion of the skin (Fig. 2.5). If fungation does not occur, there may be widespread skin involvement leading to thickening and a typical pattern of irregularity termed “peau d’orange” (Fig. 2.6). In this instance there is local lymphatic infiltration and obstruction leading to edema.
Figure 2.4 Calcification seen in mammography image of breast of patient who subsequently underwent excision of carcinoma of the breast.
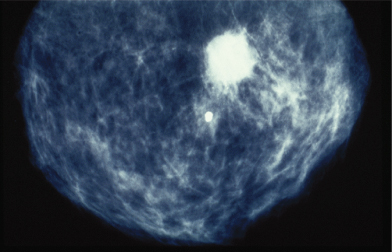
Presentations may occur, less commonly, from sites of metastases and include the detection of an enlarged lymph node or symptoms from distant metastases to bone, brain, or liver. These may cause pain and neurological symptoms and signs. As with all malignancies, nonspecific symptoms, including anorexia and lethargy, may predominate.
Figure 2.5 Fungating breast carcinoma. The primary tumor grew locally and eventually invaded the skin, causing local fungation.
Diagnostic Procedures and Investigations
Physical signs associated with breast cancer include the presence of a firm irregular mass and fixation to skin or deep structures such as the chest wall. Mammography is widely used in the initial assessment of lumps thought to be possible breast cancer; the typical X-ray appearances are fine calcification with areas of irregularity. Ultimately, diagnosis depends on a histological or cytological assessment of a specimen from the mass. Fine-needle aspiration usually provides sufficient cells for cytological assessment but a core biopsy is often obtained using a percutaneous biopsy needle which will yield sufficient tissue for histological assessment. Occasionally, the diagnosis remains uncertain and an excision biopsy may be required.
Figure 2.6 Typical features of “peau d’orange” of the breast, with thickening of the skin from edema and puckering of the skin. A large underlying tumor could be felt.
In breast cancer, the importance of obtaining cells extends well beyond that of simply confirming the diagnosis of malignancy. It is also now an exemplar of the great potential of molecular phenotyping of cancer tissues for disease subclassification and also for individualizing therapeutics. The first successful application for molecular diagnosis in breast cancer was the detection of the presence of hormone receptors – both progesterone and estrogen. This is already exploited in clinical practice, because positive receptor status correlates with the likelihood of benefit from the use of hormone manipulation therapy, as exemplified by tamoxifen. The molecular taxonomy of breast cancer now also includes detecting the presence of oncogenic HER2 receptor (found in 20–30% of invasive breast cancers). HER2 status not only improves prognostic information as it is associated with increased risk of disease recurrence and reduced survival, but importantly also clearly defines a subset of patients who will benefit most from use of therapies targeting this protein, such as trastuzumab. The importance of these tissue markers is emphasized by the description of those breast cancers that do not express either hormone receptors or HER2 mutations as triple negative breast cancers. It is likely that other molecular markers will be increasingly employed to assist choice of patients for other drugs, such as PARP inhibitors (see also discussion of synthetic lethality in Chapter 10). The use of genetic testing in familial cancer syndromes is discussed elsewhere.
Treatment decisions are based on the histological grade and size of the primary tumor, together with the presence or absence of metastases. The vast majority of patients will undergo resection of the primary tumor, and preoperative assessment of the extent of disease varies between centers. Basic investigations, including chest X-ray, liver function tests, and full blood count, would be considered standard and provide an indication of possible metastases. Most centers also perform abdominal imaging to exclude liver metastases. This may be with CT or ultrasound scanning. The presence of bone pain with elevated serum calcium or alkaline phosphatase indicate the possibility of bone metastases that can be detected with isotope bone scans. Imaging of the brain should only be performed routinely in patients who complain of symptoms suggesting possible cerebral metastases. The TNM staging system is widely used alongside the histological grade of tumor and receptor status when deciding on appropriate therapy (see Table 2.2).
Table 2.2 TNM staging system for breast cancer
| Stage | Tumor description |
| T1 | Tumor less than 2 cm in diameter |
| T2 | Tumor 2–5 cm in diameter |
| T3 | Tumor more than 5 cm |
| T4 | Tumor of any size with direct extension to chest wall or skin |
| N0 | No palpable node involvement |
| N1 | Mobile ipsilateral node(s) |
| N2 | Fixed ipsilateral nodes |
| N3 | Supraclavicular or infraclavicular nodes or edema of arm |
| M0 | No distant metastases |
| M1 | Distant metastases |
Treatment
Surgery
Surgical resection of primary breast tumors aims to control local disease and prevent recurrence in regional draining lymph nodes. Our understanding of the biology of breast cancer is a major example of how clinical and fundamental research have modified a therapeutic approach. Until relatively recently, the surgical approach to breast cancer was to undertake a radical operation with removal of the breast, block dissection of the axillary nodes, and often removal of underlying muscle. Radical mastectomy is a mutilating and disfiguring operation that often led to marked long-term morbidity. However, once it was recognized that blood-borne metastases and not lymphatic and local spread were the main causes of dissemination, the role of surgery was adapted. In particular, since most patients die from metastases that may have been “seeded” prior to the opportunity for surgery, one might expect only a limited benefit from aggressively removing the primary tumor, surrounding tissues, and lymph nodes.
Today, surgery is less radical, with the widespread adoption of breast-conserving surgical approaches, such as lumpectomy (removal of the local tumor alone) when possible. The choice of surgery is affected by the size of the primary tumor and of the breast (large tumors in small breasts are often removed with better cosmetic results using a simple mastectomy than with lumpectomy) and the presence of multiple tumors within the same breast or widespread intraduct carcinoma (both providing indications for mastectomy rather than lumpectomy). Surgical reconstruction is now increasingly used for patients who have undergone mastectomy. With modern techniques, this can produce excellent cosmetic results but one concern with immediate reconstructuion is that if subsequent radiotherapy is required, increased cardiac irradiation may occur in left-sided cancers.
Radiotherapy
Conservative surgery alone is followed by local recurrence in approximately 30% of cases, but can be reduced to just 5–10% by employing postoperative radiotherapy. Recurrence is more common with tumors >5 cm in diameter or in patients with axillary node disease. The results from the United States (where radical mastectomy is still more widely used than in Europe) suggest local recurrence rates following radical surgery alone are 4–14%, indicating the equivalence with breast-conserving surgery followed by radiotherapy. Likewise, survival appears to be identical in the two groups and these results strongly support the use of breast-conserving surgery with postoperative radiotherapy whenever technically possible. Interestingly (and perhaps not surprising when considering the biology of the disease), the vast majority of randomized studies have shown no effect on survival of postoperative radiotherapy, despite a reduction in local recurrence rates. A meta-analysis (pooled statistical analysis of multiple single studies) which included 8000 patients did suggest a slight, but significant, improvement in survival for the radiotherapy arm, but these results must be interpreted with some caution, particularly as no single prospective study has demonstrated this. Meta-analyses are particularly prone to bias, because negative studies may not have been reported in the literature (and thus self-evidently are not available for the meta-analysis) and because there may be considerable variation between groups of patients in the different studies included.
For patients with large primary tumors (particularly those >5 cm or with direct extension to the chest wall or skin) and for a large proportion of patients with clear nodal involvement, surgery appears to have little role other than occasionally to debulk the tumor for symptomatic benefits. For such patients, radiotherapy has an important role, producing high local control rates (80–90%). It is usually given in combination with hormones and/or chemotherapy.
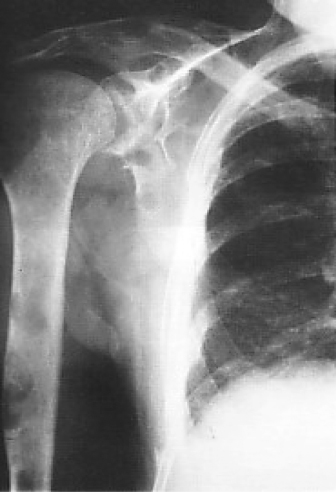
As with all malignancies, radiotherapy can be particularly useful for patients who have developed painful areas of metastasis. This is particularly the case with bone lesions, where symptomatic benefit is achieved in the majority of patients (Fig. 2.7). Between 15% and 20% of patients will develop cerebral metastases, and radiotherapy can reduce tumor volume and improve symptoms in the majority of these patients. Likewise skin, lymph node, and other areas of metastasis can benefit from local radiotherapy. One final role for radiotherapy is the induction of menopause with pelvic irradiation. This appears to be as valuable as oophorectomy for the relief of symptoms from metastatic disease if patients are premenopausal.
Figure 2.7 Plain X-ray showing extensive lytic metastases in the humerus of a patient with carcinoma of the right breast. This caused severe pain, requiring local radiotherapy and subsequent bisphosphonate therapy.
Provided by Natalie Mahowald.
Chemotherapy
Breast cancer is a relatively chemosensitive disease, with numerous single agents producing responses in 30–50% of patients. The anthracylines, taxanes, alkylating agents, and vinca alkaloids are most widely utilized, generally in combinations. More recently, capecitabine and gemcitabine have been used in prospective trials, commonly in combination with paclitaxel. A large proportion of patients will receive chemotherapy following a diagnosis of breast cancer, in either the adjuvant (immediate postoperative) or the metastatic setting. Several trials are examining the role of chemotherapy in the preoperative, neoadjuvant setting. This approach attempts to treat any micrometastatic disease early and potentially reduce the need for radical surgery. It now has an established role in some patients with highly aggressive locally advanced and inflammatory breast cancers. There is a proven role for chemotherapy in the adjuvant and metastatic settings.
The role of adjuvant therapy is based on the observation that even at relatively early stages of development, a proportion of patients with breast cancer already have micrometastases. In fact, when taken together with the demonstration of large numbers of circulating tumor cells (CTCs) in a patient’s blood, and the demonstration of metastases-related gene expression in early primary tumors, which are present before overt metastatic disease, these observations are having a profound impact on how we think about metastases (see Chapter 12).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


