Chapter Fifty-Four. The breasts and lactation
Introduction
Lactation is the production of milk by specialised organs called mammary glands, named from the Latin word mamma for breast. Humans are classified as mammals and distinguished from other vertebrates because of their ability to produce milk for their young. Lactation was probably a key physiological feature that enabled mammals to survive the climatic changes that led to the demise of the dinosaurs about 65 million years ago (Czerkas & Czerkas 1990).
The human mammary gland, an exocrine gland, is the only organ not fully developed at birth. Dramatic changes occur in size, shape and function in the mammary glands from birth through pregnancy, lactation and ultimately involution. It is essential that all practitioners involved with women during pregnancy and childbirth have sound knowledge and understanding of the anatomy of the human breast and the physiological mechanisms of milk production. This knowledge will help them to encourage and support women to breastfeed their babies.
The anatomy of the breast
Situation, shape and size
The adult breasts are always paired and develop bilaterally on the ventral surface of the body. They possibly originate from modified apocrine sweat glands and subsequently form part of the skin. The shape of the breast varies from woman to woman but it tends to be dome-shaped in adolescence, becoming more hemispheric and finally pendulous in parous females (Lawrence & Lawrence 2005). The two breasts are situated on the anterior chest wall on either side of the midline and will vary in size depending on the amount of adipose tissue present. The mature breast extends between the second rib and the sixth intercostal cartilage and lies over the pectoralis major muscle from sternum to axilla. Mammary glandular tissue projects somewhat into the axillary region blending with the anterior axillary fold and this is known as the tail of Spence. The nipple is surrounded by areola and protrudes from the centre of each breast at the level of the fourth intercostal space.
Structure
The mammary glands are modified exocrine glands comprising skin, subcutaneous tissue and the corpus mammae (body of the breast). The corpus mammae is the breast mass remaining after the breast is freed from the deep attachments and the skin, subcutaneous connective tissue and adipose tissue are removed.
Corpus mammae (body of the breast)
The tissue of the mammary gland consists of two major divisions: the parenchyma and the stroma. The parenchyma or glandular (secretory) tissue is the functional component of the breast tissue. The stroma comprises adipose (fatty) tissue, blood and lymph vessels, nerve tissue and is supported by a loose framework of fibrous connective tissue called Cooper’s ligaments.
The parenchyma consists of epithelial glandular tissue with an extensive system of branching ducts (Fig. 54.1B). The traditional description of breast anatomy has recently been replaced by findings emerging from recent research studies (Table 54.1).
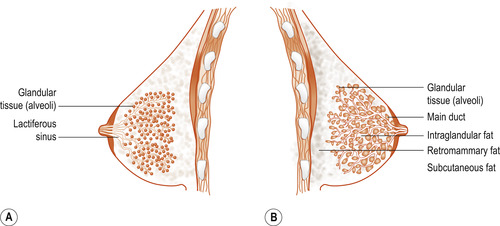 |
| Figure 54.1 (A) Traditional schematic diagram of the anatomy of the breast. The main milk ducts below the nipple are depicted as dilated portions of lactiferous sinuses and the glandular tissue within the breast. (B) Schematic diagram of the ductal anatomy of the breast based on the findings of Ramsay et al (2005). Milk ducts are shown to be small and branch a short distance from the base of the nipple. The ductal system is erratic and glandular tissue is situated directly beneath the nipple. (Reproduced with permission from Geddes 2007.) |
| Traditional descriptions | Current descriptions (based on recent research findings) |
|---|---|
| Approx. 22 ducts leading to the nipple | Approx. 9 ducts (range 4–18) in each lactating breast (Ramsay et al 2005) |
| 15–20 lobes with each lobe being a separate entity and arranged in a structured way radiating out from the nipple | Lobes are merged and difficult to separate surgically (Ohtake et al 2001). Ducts are not always arranged systematically in a radial pattern and main ducts may lie under one another (Geddes 2007) |
| Alveoli connect to very small ducts that join to form larger ducts draining the lobules. Larger ducts finally merge into one milk duct for each lobe | Duct diameter emanates from the glandular tissue directly under the nipple. Duct enlargement occurs at points where multiple branches merge and ducts in the periphery of the breast are sometimes the same size as those near the nipple (Ramsay et al 2005) |
| Lactiferous ampulla (reservoir for milk). This milk is available to the infant before and during suckling | There is no evidence of an ampulla (reservoir for milk). Only small amounts of milk (1–10 ml) can be expressed prior to milk ejection (Kent et al 2002) and the infant consumes little milk prior to milk ejection (Ramsay et al 2004) The smaller number, size and shape of ducts observed suggest that the main function of the ducts is the transport rather than the storage of milk. Milk ducts at the base of the nipple are superficial and easily compressed (Ramsay et al 2004) |
| Predominantly glandular tissue and the proportion of glandular tissue increase relative to the adipose tissue. Calculated ratio of glandular tissue to adipose tissue is 2:1 for lactating women compared with 1:1 for non-lactating women (Heggie 1996, Jamal et al 2004) | Some women have an abundance of adipose tissue and it may consititute half the breast tissue There is a large amount of glandular tissue (2.5 times more) relative to adipose tissue close to the base of the nipple (approx. 2.5 times as much) (Ramsay et al 2005) |
The alveoli, ducts and lobes
As with all exocrine glands, the glandular tissue contains secretory and ductal tissue within a lobuloalveolar system (Fig. 54.2). Lobes are merged together and difficult to separate surgically (Ohtake et al 2001). The basic glandular unit, making up a lobe, consists of alveoli connected to ducts leading to the nipple. The alveolus is the site of milk synthesis and secretion and consists of clusters of epithelial secretory cells (lactocytes) surrounded by myoepithelial cells to form smooth muscle contractile units responsible for ejecting milk into the ducts from the lumen of the alveoli. Ductal epithelial cells line the interior of the branching ductal network in a single layer. Lactocytes secrete all the components of breast milk into the alveolar lumen. Ductal cells are of the same family as lactocytes and comprise many of the same structural and membrane-bound proteins. They are, however, less functionally complex than lactocytes in that these cells do not have the ability to synthesise milk. A recent study found that each breast contains about 9 milk ducts (range 4–18 ducts) arranged in a complex network and which converge at the nipple (Ramsay et al 2005).
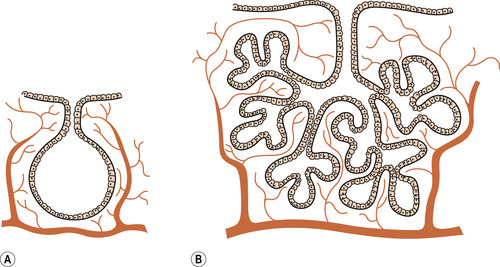 |
| Figure 54.2 The structure of the breast. (A) Single alveolus. (B) One lobule. (From Henderson C, Macdonald S 2004, with kind permission of Elsevier.) |
The basal lamina
Each alveolus is surrounded by a basement membrane (basal lamina) made up of collagen, glycoprotein and glycosaminoglycans secreted by the epithelial cells where they are in contact with connective tissue. This provides a barrier between the epithelial and stromal components of breast tissue which cannot be crossed by cells other than leucocytes. Lymphocytes or monocytes are found wedged between the secretory cells of the alveoli and have migrated there. They play a role in local production of antibodies in the form of immunoglobulin A (IgA) for secretion into the breast milk.
The secretory cells
The secretory cells lining the alveoli are cuboidal and very polarised in structure (Fig. 54.3). The nucleus is situated at the base of the cell facing the circulation, and facing the lumen is a well-developed Golgi apparatus with layers of flattened vesicles. Most of the cell is occupied by rough endoplasmic reticulum and there are a large number of mitochondria. There are large fat droplets and vesicles containing granules of protein. The basal surface of the cell has numerous infoldings for the uptake of substrate for milk production while the surface facing the lumen is covered with microvilli for secretion of milk.
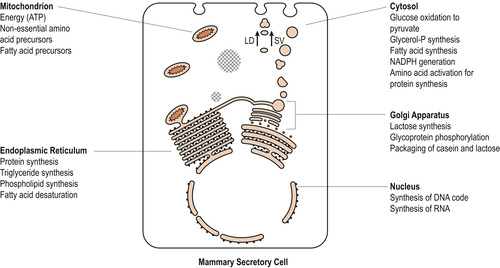 |
| Figure 54.3 Schematic representation of cytological and biochemical interrelationships of secretory cell of mammary gland. LD, liquid droplet; SV, secretory vesicle. (Reproduced with permission from Lawrence & Lawrence 2005.) |
The stroma
Connective and adipose tissue, which form the largest part of the mammary glands in the non-pregnant state, separate and support the glandular tissue. The breasts are held in position by the suspensory ligaments of Astley Cooper which form from the interlobar connective tissue. These fibrous bands of tissue attach the breast to the underlying muscle fascia and to the overlying dermis.
The nipple and areola
The skin of the breast includes the nipple and surrounding areola which are all visible externally. The nipple or papilla mammae is a conic elevation located in the centre of the areola. The nipple contains about 4–18 milk ducts surrounded by a muscular fibroelastic tissue. The smooth muscle fibres in the nipple represent a closing mechanism for the milk ducts and the nipple is richly innervated with sensory nerve endings. Nipple erection is induced by tactile, sensory or autonomic sympathetic stimuli. Local venostasis and hyperaemia occur to enhance the process of erection. The nipple and areola are extremely elastic due to the muscular fibroelastic system which functions to decrease the surface of the areola, produce nipple erection and empties the lactiferous sinuses during lactation. When the nipple erects, it becomes smaller, firmer and more prominent.
The areola, or areola mammae, is a circular pigmented area surrounding the nipple. Within the areola are about 18 sebaceous and lactiferous glands known as Montgomery’s tubercles. These glands provide secretions to lubricate and protect the areola and nipple during pregnancy and lactation (Inch 2003).
Blood supply
The internal mammary artery (60%) and the lateral mammary branch of the lateral thoracic artery (30%) provide the major blood supply, with smaller sources of arterial blood from the posterior intercostal arteries and the pectoral branch of the thoracoacromial artery (Hirsch et al 1995). The venous supply parallels the arterial supply and bears similar names. Veins end in the internal thoracic and axillary veins and create an anastomotic circle called the circulus venosus around the base of the papilla behind the nipple.
Lymphatic vessels
There is an extensive lymphatic drainage system forming a plexus beneath the areola and between the lobes of the breast with free communication between the two breasts. There are two main pathways by which lymph is drained from the breast and include the axillary nodes and the internal mammary nodes. Lymph from both the medial and lateral portions of the breast drains to the axillary nodes (75%) whereas the internal mammary nodes receive lymph from the deep portion of the breast.
Nerve supply
The breast is innervated primarily by branches from the fourth, fifth and sixth intercostal nerves. The nerve supply to the corpus mammae is sparse and contains only sympathetic nerves accompanying blood vessels. The sensory innervation of the nipple and areola is extensive and consists of both sensory and sympathetic autonomic nerves:
• Somatic sensory nerves convey impulses from skin receptors to the central nervous system.
• Sympathetic (efferent) nerves innervate blood vessels and the contractile muscles of the nipple.
Development of the breast
The mammary gland undergoes three major phases of growth and development before pregnancy and lactation: in utero; during the first 2 years of life; and at puberty. Embryogenesis refers to the embryonic development of the organ in utero. Mammogenesis refers to the growth and development of the mammary glands. This stage occurs in two phases as the glands respond first to the hormones of puberty and then later to the hormones of pregnancy. Lactogenesis refers to the initiation and production of milk.
Early development and puberty
In the 4th week of embryonic life, a primitive milk streak develops from axilla to groin on the trunk of the embryo (2.5 mm long at this stage). This streak becomes the mammary ridge or milk line by the 5th week. The ridge is actually a thickening of epithelial tissue and is accompanied by growth inward at the chest wall, which will be the region of the future gland.
Embryogenesis of the mammary glands begins in the 6-week embryo and proliferation continues until milk ducts are developed by the time of birth. During this time, processes of dividing and branching take place, giving rise to the future lobes and lobules and much later to the alveoli. Specialised cells differentiate into breast structures such as nipple, areola, glands, hair follicles and Montgomery glands. Development is influenced by the placental sex hormones between 28 and 32 weeks to stimulate the formation of channels (canalisation). From 32 to 40 weeks of gestation, lobular–alveolar structures containing colostrum develop. During this time, the fetal mammary glands increase four times and the nipple and areola further develop and become pigmented. After birth, the neonate may secrete colostrum known as ‘witch’s milk’.
Mammary gland development during childhood merely keeps pace with physical growth. At puberty, oestrogen becomes the major influence on female breast development. Under the influence of oestrogen, primary and secondary ducts grow and divide and form terminal end buds which develop into new branches and later become alveoli in the mature breast. During each menstrual cycle, proliferation and active growth of duct tissue occurs during the follicular and ovulatory phases, reaching a maximum in the late luteal phase before regressing. Complete development of mammary function occurs only in pregnancy.
Development in pregnancy
The breast reaches its full functional capacity at lactation and, as a result, several internal and external changes occur (Geddes 2007). Changes in levels of circulating hormones result in profound changes in ductular–lobular–alveolar growth during pregnancy (Fig. 54.4). Breasts begin to exhibit changes at about the 6th week of pregnancy and may be useful in confirming pregnancy (Blackburn 2007).
1. Early in pregnancy, the luteal and placental hormones are responsible for a marked increase in the development of the duct system and formation of lobes. Placental lactogen, prolactin and chorionic gonadotrophin contribute to the accelerated growth. Oestrogen is responsible for development in the duct system and progesterone is responsible for lobular formation (Lawrence & Lawrence 2005). This results in the breasts feeling nodular and lumpy and the woman may feel the breasts tender and tingly. Growth continues throughout pregnancy and the breasts increase in size.
2. Prolactin is produced by the anterior lobe of the pituitary gland and this hormone is essential for the complete lobular–alveolar development. It influences the alveolar cells to initiate milk secretion and stimulates the glandular production of colostrum. The prolactin moves through the blood from the central nervous system into the prolactin receptor sites in the lactocytes. Prolactin then transmits a ‘message’ to the lactocytes to begin production of milk. By the 2nd trimester, colostrum is secreted under the influence of placental lactogen (about 16 weeks). During pregnancy, prolactin is prevented from exerting its effect on milk excretion by the high circulating levels of progesterone.
3. Vascularity increases and the appearance of a network of subcutaneous veins is visible beneath the skin. This network increases in size and complexity throughout pregnancy.
4. By 12 weeks the nipples are now more prominent and the areola develops an increased fullness and brown pigmentation called the primary areola of pregnancy. Montgomery’s tubercles further develop and become more prominent and appear as raised projections. The dark pigmentation of the areola may be a visual signal for the newborn baby to find the nipple (Lawrence & Lawrence 2005).
5. By the 24th week, there is further pigmentation around the primary areola known as the secondary areola. This is especially noticeable in dark-haired people.
6. By term the breasts usually enlarge by 5 cm overall and increase by 1400 g in weight.
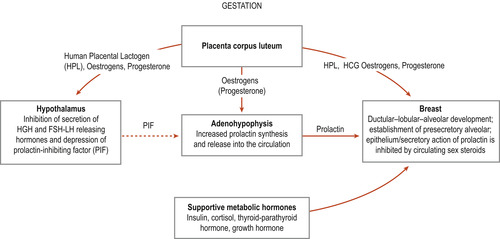 |
| Figure 54.4 Hormonal preparation of breast during pregnancy for lactation. (Reproduced with permission from Lawrence & Lawrence 2005.) |
Maternal nutrition and lactation
During pregnancy, energy is stored in the form of body fat and mainly deposited on the trunk and legs. In women with adequate nutrition, this portion of the weight gain of pregnancy amounts to 4 kg, which is equivalent to an energy store of 35 000 kcal. This will provide for 4 months of lactation and 300 kcal/day for the baby, enough to ensure survival if the mother is deprived of food as may happen in famine conditions. If a woman does not breastfeed, these fat stores may be difficult to remove, contributing to obesity as successive pregnancies deposit their stores (Baker et al 2008). Lactating women are much more likely to regain their figures.
There are two physiological aids to the accumulation of these fat stores:
1. The effect of progesterone and other hormones on the metabolism during pregnancy.
2. The slowing down of energy usage as pregnancy proceeds.
After delivery of the baby, these extra stored calories are converted into milk. The recommendation of an additional 500 kcal/day is now considered to be the upper level for lactating women (Riordan 2005). The conversion of calorie intake to milk is very efficient. Women with marginal nutrition (below 1800 kcal/day), such as those in developing countries, are able to breastfeed for at least 6 months or more. This is due to the enhanced ability to store energy in pregnancy coupled with the highly efficient conversion of food energy to breast milk.
The physiology of lactation
Lactation is the physiological completion of the reproductive cycle (Lawrence & Lawrence 2005). The process of lactation can be divided into three stages during which human milk varies in components, appearance and volume:
1. Lactogenesis I: the initiation of milk secretion.
2. Lactogenesis II: the production of colostrum and transitional milk.
3. Lactogenesis III: the development of milk and the maintenance of established lactation.
Lactogenesis
There are important factors in the initiation of the cascade of events necessary to ensure that a supply of milk is readily available for the baby at birth. These include the preparation of mammary epithelium, the withdrawal of progesterone, the maintenance of prolactin levels and the removal of milk from the breast after birth (Neville et al 2001). This is achieved through the influences and control of the processes involved in lactogenesis (Table 54.2).
| Stage | Developments |
|---|---|
| Lactogenesis I | |
| Begins when milk components are first seen in breast tissue and colostrum can be expressed from the breast during pregnancy (normally from mid-pregnancy until day 2 after birth) | |



