274 | The Bradyarrhythmias: Disorders of the Sinoatrial Node |
Electrical activation of the heart normally originates in the sinoatrial (SA) node, the predominant pacemaker. Other subsidiary pacemakers in the atrioventricular (AV) node, specialized conducting system, and muscle may initiate electrical activation if the SA node is dysfunctional or suppressed. Typically, subsidiary pacemakers discharge at a slower rate and, in the absence of an appropriate increase in stroke volume, may result in tissue hypoperfusion.
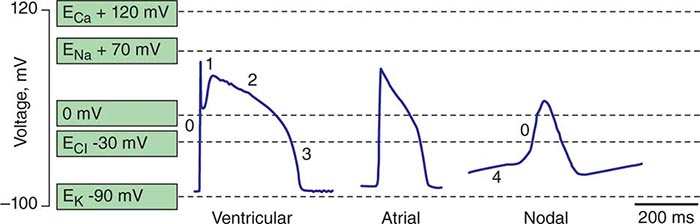
Spontaneous activation and contraction of the heart are a consequence of the specialized pacemaking tissue in these anatomic locales. As described in Chap. 273e, action potentials in the heart are regionally heterogeneous. The action potentials in cells isolated from nodal tissue are distinct from those recorded from atrial and ventricular myocytes (Fig. 274-1). The complement of ionic currents present in nodal cells results in a less negative resting membrane potential compared with atrial or ventricular myocytes. Electrical diastole in nodal cells is characterized by slow diastolic depolarization (phase 4), which generates an action potential as the membrane voltage reaches threshold. The action potential upstrokes (phase 0) are slow compared with atrial or ventricular myocytes, being mediated by calcium rather than sodium current. Cells with properties of SA and AV nodal tissue are electrically connected to the remainder of the myocardium by cells with an electrophysiologic phenotype between that of nodal cells and that of atrial or ventricular myocytes. Cells in the SA node exhibit the most rapid phase 4 depolarization and thus are the dominant pacemakers in a normal heart.
FIGURE 274-1 Action potential profiles recorded in cells isolated from sinoatrial or atrioventricular nodal tissue compared with those of cells from atrial or ventricular myocardium. Nodal cell action potentials exhibit more depolarized resting membrane potentials, slower phase 0 upstrokes, and phase 4 diastolic depolarization.
Bradycardia results from a failure of either impulse initiation or impulse conduction. Failure of impulse initiation may be caused by depressed automaticity resulting from a slowing or failure of phase 4 diastolic depolarization (Fig. 274-2), which may result from disease or exposure to drugs. Prominently, the autonomic nervous system modulates the rate of phase 4 diastolic depolarization and thus the firing rate of both primary (SA node) and subsidiary pacemakers. Failure of conduction of an impulse from nodal tissue to atrial or ventricular myocardium may produce bradycardia as a result of exit block. Conditions that alter the activation and connectivity of cells (e.g., fibrosis) in the heart may result in failure of impulse conduction.
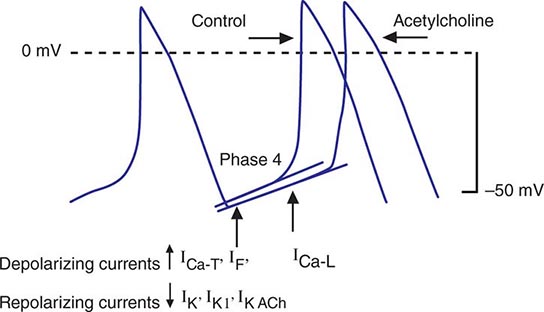
FIGURE 274-2 Schematics of nodal action potentials and the currents that contribute to phase 4 depolarization. Relative increases in depolarizing L- (ICa-L) and T- (ICa-T) type calcium and pacemaker currents (If) along with a reduction in repolarizing inward rectifier (IK1) and delayed rectifier (IK) potassium currents result in depolarization. Activation of ACh-gated (IKACh) potassium current and beta blockade slow the rate of phase 4 and decrease the pacing rate. (Modified from J Jalife et al: Basic Cardiac Electrophysiology for the Clinician, Blackwell Publishing, 1999.)
SA node dysfunction and AV conduction block are the most common causes of pathologic bradycardia. SA node dysfunction may be difficult to distinguish from physiologic sinus bradycardia, particularly in the young. SA node dysfunction increases in frequency between the fifth and sixth decades of life and should be considered in patients with fatigue, exercise intolerance, or syncope and sinus bradycardia.
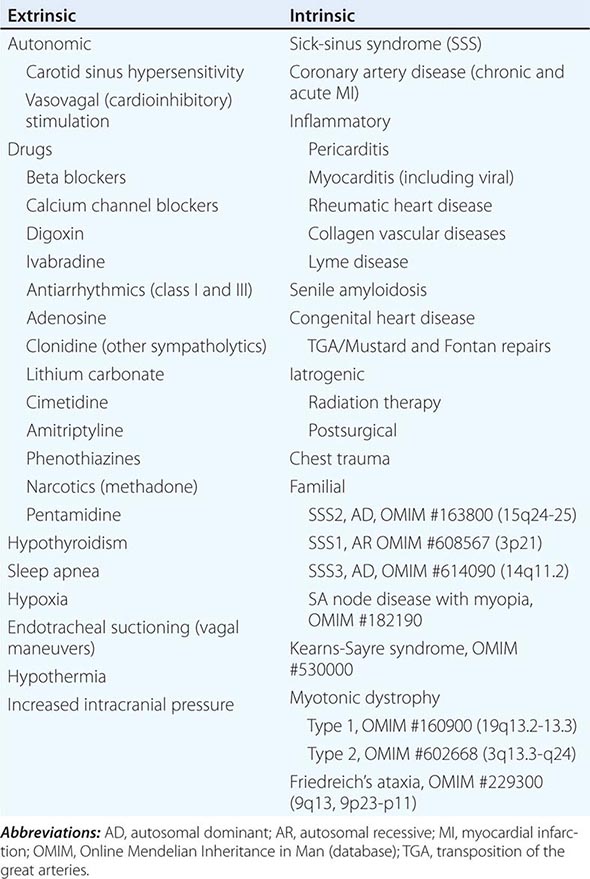
Permanent pacemaking is the only reliable therapy for symptomatic bradycardia in the absence of extrinsic and reversible etiologies such as increased vagal tone, hypoxia, hypothermia, and drugs (Table 274-1). Approximately 50% of the 150,000 permanent pacemakers implanted in the United States and 20–30% of the 150,000 of those in Europe were implanted for SA node disease.
ETIOLOGIES OF SA NODE DYSFUNCTION |
STRUCTURE AND PHYSIOLOGY OF THE SA NODE
The SA node is composed of a cluster of small fusiform cells in the sulcus terminalis on the epicardial surface of the heart at the right atrial–superior vena caval junction, where they envelop the SA nodal artery. The SA node is structurally heterogeneous, but the central prototypic nodal cells have fewer distinct myofibrils than does the surrounding atrial myocardium, no intercalated disks visible on light microscopy, a poorly developed sarcoplasmic reticulum, and no T-tubules. Cells in the peripheral regions of the SA node are transitional in both structure and function. The SA nodal artery arises from the right coronary artery in 55–60% and the left circumflex artery in 40–45% of persons. The SA node is richly innervated by sympathetic and parasympathetic nerves and ganglia.
Irregular and slow propagation of impulses from the SA node can be explained by the electrophysiology of nodal cells and the structure of the SA node itself. The action potentials of SA nodal cells are characterized by a relatively depolarized membrane potential (Fig. 274-1) of –40 to –60 mV, slow phase 0 upstroke, and relatively rapid phase 4 diastolic depolarization compared with the action potentials recorded in cardiac muscle cells. The relative absence of inward rectifier potassium current (IK1) accounts for the depolarized membrane potential; the slow upstroke of phase 0 results from the absence of available fast sodium current (INa) and is mediated by L-type calcium current (ICa-L); and phase 4 depolarization is a result of the aggregate activity of a number of ionic currents. Prominently, both L- and T-type (ICa-T) calcium currents, the pacemaker current (so-called funny current, or If) formed by hyperpolarization-activated cyclic nucleotide-gated channels, and the electrogenic sodium-calcium exchanger provide depolarizing current that is antagonized by delayed rectifier (IKr) and acetylcholine-gated (IKACh) potassium currents. ICa-L, ICa-T, and If are modulated by β-adrenergic stimulation and IKACh by vagal stimulation, explaining the exquisite sensitivity of diastolic depolarization to autonomic nervous system activity. The slow conduction within the SA node is explained by the absence of INa and poor electrical coupling of cells in the node, resulting from sizable amounts of interstitial tissue and a low abundance of gap junctions. The poor coupling allows for graded electrophysiologic properties within the node, with the peripheral transitional cells being silenced by electrotonic coupling to atrial myocardium.
ETIOLOGY OF SA NODAL DISEASE
SA nodal dysfunction has been classified as intrinsic or extrinsic. The distinction is important because extrinsic dysfunction is often reversible and generally should be corrected before pacemaker therapy is considered (Table 274-1). The most common causes of extrinsic SA node dysfunction are drugs and autonomic nervous system influences that suppress automaticity and/or compromise conduction. Other extrinsic causes include hypothyroidism, sleep apnea, and conditions likely to occur in critically ill patients such as hypothermia, hypoxia, increased intracranial pressure (Cushing’s response), and endotracheal suctioning via activation of the vagus nerve.
Intrinsic sinus node dysfunction is degenerative and often is characterized pathologically by fibrous replacement of the SA node or its connections to the atrium. Acute and chronic coronary artery disease (CAD) may be associated with SA node dysfunction, although in the setting of acute myocardial infarction (MI; typically inferior), the abnormalities are transient. Inflammatory processes may alter SA node function, ultimately producing replacement fibrosis. Pericarditis, myocarditis, and rheumatic heart disease have been associated with SA nodal disease with sinus bradycardia, sinus arrest, and exit block. Carditis associated with systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and mixed connective tissue disorders (MCTDs) may also affect SA node structure and function. Senile amyloidosis is an infiltrative disorder in patients typically in the ninth decade of life; deposition of amyloid protein in the atrial myocardium can impair SA node function. Some SA node disease is iatrogenic and results from direct injury to the SA node during cardiothoracic surgery.
Rare heritable forms of sinus node disease have been described, and several have been characterized genetically. Autosomal dominant sinus node dysfunction in conjunction with supraventricular tachycardia (i.e., tachycardia-bradycardia variant of sick-sinus syndrome [SSS2]) has been linked to mutations in the pacemaker current (If) subunit gene HCN4 on chromosome 15. An autosomal recessive form of SSS1 with the prominent feature of atrial inexcitability and absence of P waves on the electrocardiogram (ECG) is caused by mutations in the cardiac sodium channel gene, SCN5A, on chromosome 3. Variants in myosin heavy chain 6 (MYH6) increase the susceptibility to SSS (SSS3). SA node dysfunction associated with myopia has been described but not genetically characterized. There are several neuromuscular diseases, including Kearns-Sayre syndrome (ophthalmoplegia, pigmentary degeneration of the retina, and cardiomyopathy) and myotonic dystrophy, that have a predilection for the conducting system and SA node.
SSS in both the young and the elderly is associated with an increase in fibrous tissue in the SA node. The onset of SSS may be hastened by coexisting disease, such as CAD, diabetes mellitus, hypertension, and valvular diseases and cardiomyopathies.
CLINICAL FEATURES OF SA NODE DISEASE
SA node dysfunction may be completely asymptomatic and manifest as an ECG anomaly such as sinus bradycardia; sinus arrest and exit block; or alternating supraventricular tachycardia, usually atrial fibrillation, and bradycardia. Symptoms associated with SA node dysfunction, in particular tachycardia-bradycardia syndrome, may be related to both slow and fast heart rates. For example, tachycardia may be associated with palpitations, angina pectoris, and heart failure, and bradycardia may be associated with hypotension, syncope, presyncope, fatigue, and weakness. In the setting of SSS, overdrive suppression of the SA node may result in prolonged pauses and syncope upon termination of the tachycardia. In many cases, symptoms associated with SA node dysfunction result from concomitant cardiovascular disease. A significant minority of patients with SSS develop signs and symptoms of heart failure that may be related to slow or fast heart rates.
One-third to one-half of patients with SA node dysfunction develop supraventricular tachycardia, usually atrial fibrillation or atrial flutter. The incidence of persistent atrial fibrillation in patients with SA node dysfunction increases with advanced age, hypertension, diabetes mellitus, left ventricular dilation, valvular heart disease, and ventricular pacing. Remarkably, some symptomatic patients may experience an improvement in symptoms with the development of atrial fibrillation, presumably from an increase in their average heart rate. Patients with the tachycardia-bradycardia variant of SSS, similar to patients with atrial fibrillation, are at risk for thromboembolism, and those at greatest risk, including patients ≥65 years and patients with a prior history of stroke, valvular heart disease, left ventricular dysfunction, or atrial enlargement, should be treated with anticoagulants. Up to one-quarter of patients with SA node disease will have concurrent AV conduction disease, although only a minority will require specific therapy for high-grade AV block.
The natural history of SA node dysfunction is one of varying intensity of symptoms even in patients who present with syncope. Symptoms related to SA node dysfunction may be significant, but overall mortality usually is not compromised in the absence of other significant comorbid conditions. These features of the natural history need to be taken into account in considering therapy for these patients.
ELECTROCARDIOGRAPHY OF SA NODE DISEASE
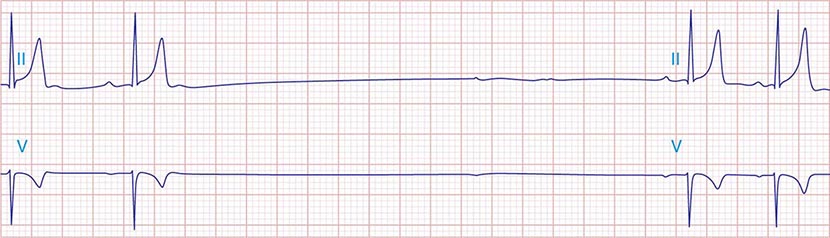
The electrocardiographic manifestations of SA node dysfunction include sinus bradycardia, sinus pauses, sinus arrest, sinus exit block, tachycardia (in SSS), and chronotropic incompetence. It is often difficult to distinguish pathologic from physiologic sinus bradycardia. By definition, sinus bradycardia is a rhythm driven by the SA node with a rate of <60 beats/min; sinus bradycardia is very common and typically benign. Resting heart rates <60 beats/min are very common in young healthy individuals and physically conditioned subjects. A sinus rate of <40 beats/min in the awake state in the absence of physical conditioning generally is considered abnormal. Sinus pauses and sinus arrest result from failure of the SA node to discharge, producing a pause without P waves visible on the ECG (Fig. 274-3). Sinus pauses of up to 3 s are common in awake athletes, and pauses of this duration or longer may be observed in asymptomatic elderly subjects. Intermittent failure of conduction from the SA node produces sinus exit block. The severity of sinus exit block may vary in a manner similar to that of AV block (Chap. 275). Prolongation of conduction from the sinus node will not be apparent on the ECG; second-degree SA block will produce intermittent conduction from the SA node and a regularly irregular atrial rhythm.
FIGURE 274-3 Sinus slowing and pauses on the electrocardiogram (ECG). The ECG is recorded during sleep in a young patient without heart disease. The heart rate before the pause is slow, and the PR interval is prolonged, consistent with an increase in vagal tone. The P waves have a morphology consistent with sinus rhythm. The recording is from a two-lead telemetry system in which the tracing labeled II mimics frontal lead II and V represents Modified Central Lead 1, which mimics lead V1 of the standard 12-lead ECG.
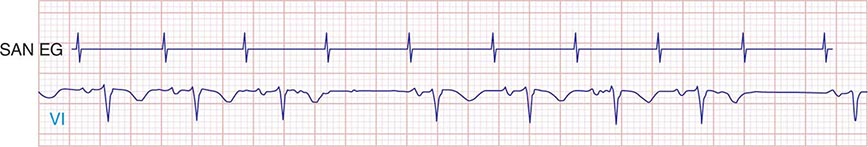
Type I second-degree SA block results from progressive prolongation of SA node conduction with intermittent failure of the impulses originating in the sinus node to conduct to the surrounding atrial tissue. Second-degree SA block appears on the ECG as an intermittent absence of P waves (Fig. 274-4). In type II second-degree SA block, there is no change in SA node conduction before the pause. Complete or third-degree SA block results in no P waves on the ECG. Tachycardia-bradycardia syndrome is manifest as alternating sinus bradycardia and atrial tachyarrhythmias. Although atrial tachycardia, atrial flutter, and atrial fibrillation may be observed, the latter is the most common tachycardia. Chronotropic incompetence is the inability to increase the heart rate in response to exercise or other stress appropriately and is defined in greater detail below.
FIGURE 274-4 Mobitz type I SA nodal exit block. A theoretical SA node electrogram (SAN EG) is shown. Note that there is grouped beating producing a regularly irregular heart rhythm. The SA node EG rate is constant with progressive delay in exit from the node and activation of the atria, inscribing the P wave. This produces subtly decreasing P-P intervals before the pause, and the pause is less than twice the cycle length of the last sinus interval.
DIAGNOSTIC TESTING
SA node dysfunction is most commonly a clinical or electrocardiographic diagnosis. Sinus bradycardia or pauses on the resting ECG are rarely sufficient to diagnose SA node disease, and longer-term recording and symptom correlation generally are required. Symptoms in the absence of sinus bradyarrhythmias may be sufficient to exclude a diagnosis of SA node dysfunction.
Electrocardiographic recording plays a central role in the diagnosis and management of SA node dysfunction. Despite the limitations of the resting ECG, longer-term recording employing Holter or event monitors may permit correlation of symptoms with the cardiac rhythm. Many contemporary event monitors may be automatically triggered to record the ECG when certain programmed heart rate criteria are met. Implantable ECG monitors permit long-term recording (12–18 months) in particularly challenging patients.
Failure to increase the heart rate with exercise is referred to as chronotropic incompetence. This is alternatively defined as failure to reach 85% of predicted maximal heart rate at peak exercise or failure to achieve a heart rate >100 beats/min with exercise or a maximal heart rate with exercise less than two standard deviations below that of an age-matched control population. Exercise testing may be useful in discriminating chronotropic incompetence from resting bradycardia and may aid in the identification of the mechanism of exercise intolerance.
Autonomic nervous system testing is useful in diagnosing carotid sinus hypersensitivity; pauses >3 s are consistent with the diagnosis but may be present in asymptomatic elderly subjects. Determining the intrinsic heart rate (IHR) may distinguish SA node dysfunction from slow heart rates that result from high vagal tone. The normal IHR after administration of 0.2 mg/kg propranolol and 0.04 mg/kg atropine is 117.2 – (0.53 × age) in beats/min; a low IHR is indicative of SA disease.
Electrophysiologic testing may play a role in the assessment of patients with presumed SA node dysfunction and in the evaluation of syncope, particularly in the setting of structural heart disease. In this circumstance, electrophysiologic testing is used to rule out more malignant etiologies of syncope, such as ventricular tachyarrhythmias and AV conduction block. There are several ways to assess SA node function invasively. They include the sinus node recovery time (SNRT), defined as the longest pause after cessation of overdrive pacing of the right atrium near the SA node (normal: <1500 ms or, corrected for sinus cycle length, <550 ms), and the sinoatrial conduction time (SACT), defined as one-half the difference between the intrinsic sinus cycle length and a noncompensatory pause after a premature atrial stimulus (normal <125 ms). The combination of an abnormal SNRT, an abnormal SACT, and a low IHR is a sensitive and specific indicator of intrinsic SA node disease.
275 | The Bradyarrhythmias: Disorders of the Atrioventricular Node |
Impulses generated in the sinoatrial (SA) node or in ectopic atrial loci are conducted to the ventricles through the electrically and anatomically complex atrioventricular (AV) node. As described in Chap. 274, the electrophysiologic properties of nodal tissue are distinct from atrial and ventricular myocardium. Cells located in the AV node sit at a relatively higher resting membrane potential than surrounding atrial and ventricular myocytes, exhibit spontaneous depolarization during phase 4 of the action potential, and have slower phase 0 depolarization (mediated by calcium influx in nodal tissue) than that seen in ventricular tissue (mediated by sodium influx).
Bradycardia may occur when conduction across the AV node is compromised, resulting in ineffective ventricular rates, with the possibility of attendant symptoms, including fatigue, syncope, and (if subsidiary pacemaker activity is insufficient) even death. It is important to recognize that in the setting of disturbed AV conduction, SA activation and atrial systole may occur at normal or even accelerated rates, while ventricular activation is either slowed or nonexistent. Transient AV conduction block is common in the young and is most likely the result of high vagal tone found in up to 10% of young adults. Acquired and persistent failure of AV conduction is decidedly rare in healthy adult populations, with an estimated incidence of 200 per million population per year. In the setting of myocardial ischemia, aging and fibrosis, or cardiac infiltrative diseases, however, persistent AV block is much more common.
As with symptomatic bradycardia arising from SA node dysfunction, permanent pacing is the only reliable therapy for symptoms arising from AV conduction block. Approximately 50% of the 150,000 permanent pacemakers implanted in the United States and 70–80% of those in Europe are implanted for disorders of AV conduction.
STRUCTURE AND PHYSIOLOGY OF THE AV NODE
The AV conduction axis is structurally complex, involving the atria and ventricles as well as the AV node. Unlike the SA node, the AV node is a subendocardial structure originating in the transitional zone, which is composed of aggregates of cells in the posterior-inferior right atrium. Superior, medial, and posterior transitional atrionodal bundles converge on the compact AV node. The compact AV node (~1 × 3 × 5 mm) is situated at the apex of the triangle of Koch, which is defined by the coronary sinus ostium posteriorly, the septal tricuspid valve annulus anteriorly, and the tendon of Todaro superiorly. The compact AV node continues as the penetrating AV bundle where it immediately traverses the central fibrous body and is in close proximity to the aortic, mitral, and tricuspid valve annuli; thus, it is subject to injury in the setting of valvular heart disease or its surgical treatment. The penetrating AV bundle continues through the annulus fibrosis and emerges along the ventricular septum adjacent to the membranous septum as the bundle of His. The right bundle branch (RBB) emerges from the distal AV bundle in a band that traverses the right ventricle (moderator band). In contrast, the left bundle branch (LBB) is a broad subendocardial sheet of tissue on the septal left ventricle. The Purkinje fiber network emerges from the RBB and LBB and extensively ramifies on the endocardial surfaces of the right and left ventricles, respectively.
The blood supply to the penetrating AV bundle is from the AV nodal artery and first septal perforator of the left anterior descending coronary artery. The bundle branches also have a dual blood supply from the septal perforators of the left anterior descending coronary artery and branches of the posterior descending coronary artery. The AV node is highly innervated with postganglionic sympathetic and parasympathetic nerves. The bundle of His and distal conducting system are minimally influenced by autonomic tone.
The cells that constitute the AV node complex are heterogeneous with a range of action potential profiles. In the transitional zones, the cells have an electrical phenotype between those of atrial myocytes and cells of the compact node (see Fig. 274-1). Atrionodal transitional connections may exhibit decremental conduction, defined as slowing of conduction with increasingly rapid rates of stimulation. Fast and slow AV nodal pathways have been described, but it is controversial whether these two types of pathway are anatomically distinct or represent functional heterogeneities in different regions of the AV nodal complex. Myocytes that constitute the compact node are depolarized (resting membrane potential ~–60 mV) and exhibit action potentials with low amplitudes, slow upstrokes of phase 0 (<10 V/s), and phase 4 diastolic depolarization; high-input resistance; and relative insensitivity to external [K+]. The action potential phenotype is explained by the complement of ionic currents expressed. AV nodal cells lack a robust inward rectifier potassium current (IK1) and fast sodium current (INa); L-type calcium current (ICa-L) is responsible for phase 0; and phase 4 depolarization reflects the composite activity of the depolarizing currents—funny current (If), ICa-L, T-type calcium current (ICa-T), and sodium calcium exchanger current (INCX)—and the repolarizing currents—delayed rectifier (IKr) and acetylcholine-gated (IKACh) potassium currents. Electrical coupling between cells in the AV node is tenuous due to the relatively sparse expression of gap junction channels (predominantly connexin-40) and increased extracellular volume.
The His bundle and the bundle branches are insulated from ventricular myocardium. The most rapid conduction in the heart is observed in these tissues. The action potentials exhibit very rapid upstrokes (phase 0), prolonged plateaus (phase 2), and modest automaticity (phase 4 depolarization). Gap junctions, composed largely of connexin-40, are abundant, but bundles are poorly connected transversely to ventricular myocardium.
ETIOLOGY OF AV CONDUCTION DISEASE
Conduction block from the atrium to the ventricle can occur for a variety of reasons in a number of clinical situations, and AV conduction block may be classified in a number of ways. The etiologies may be functional or structural, in part analogous to extrinsic and intrinsic causes of SA nodal dysfunction. The block may be classified by its severity from first to third degree or complete AV block or by the location of block within the AV conduction system. Table 275-1 summarizes the etiologies of AV conduction block. Those that are functional (autonomic, metabolic/endocrine, and drug-related) tend to be reversible. Most other etiologies produce structural changes, typically fibrosis, in segments of the AV conduction axis that are generally permanent. Heightened vagal tone during sleep or in well-conditioned individuals can be associated with all grades of AV block. Carotid sinus hypersensitivity, vasovagal syncope, and cough and micturition syncope may be associated with SA node slowing and AV conduction block. Transient metabolic and endocrinologic disturbances as well as a number of pharmacologic agents also may produce reversible AV conduction block.
ETIOLOGIES OF ATRIOVENTRICULAR BLOCK |
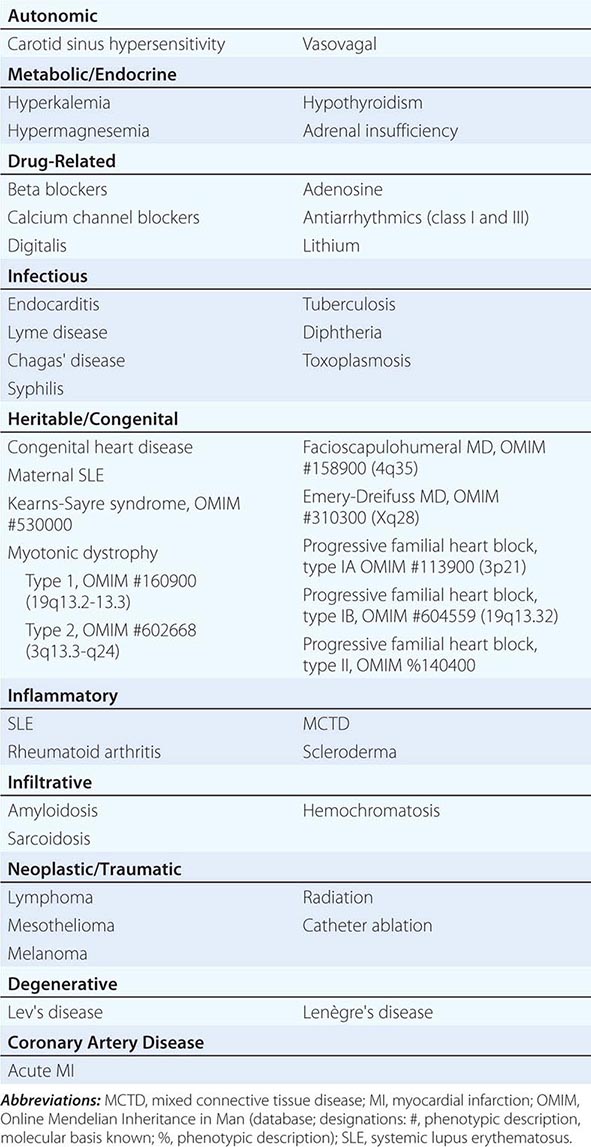
Several infectious diseases have a predilection for the conducting system. Lyme disease may involve the heart in up to 50% of cases; 10% of patients with Lyme carditis develop AV conduction block, which is generally reversible but may require temporary pacing support. Chagas’ disease, which is common in Latin America, and syphilis may produce more persistent AV conduction disturbances. Some autoimmune and infiltrative diseases may produce AV conduction block, including systemic lupus erythematosus (SLE), rheumatoid arthritis, mixed connective tissue disease, scleroderma, amyloidosis (primary and secondary), sarcoidosis, and hemochromatosis; rare malignancies also may impair AV conduction.
Idiopathic progressive fibrosis of the conduction system is one of the more common and degenerative causes of AV conduction block. Aging is associated with degenerative changes in the summit of the ventricular septum, central fibrous body, and aortic and mitral annuli and has been described as “sclerosis of the left cardiac skeleton.” The process typically begins in the fourth decade of life and may be accelerated by atherosclerosis, hypertension, and diabetes mellitus. Accelerated forms of progressive familial heart block have been identified in families with mutations in the cardiac sodium channel gene (SCN5A) and other loci that have been mapped to chromosomes 1 and 19.
AV conduction block has been associated with heritable neuromuscular diseases, including the nucleotide repeat disease myotonic dystrophy, the mitochondrial myopathy Kearns-Sayre syndrome (Chap. 462e), and several of the monogenic muscular dystrophies. Congenital AV block may be observed in complex congenital cardiac anomalies (Chap. 282), such as transposition of the great arteries, ostium primum atrial septal defects (ASDs), ventricular septal defects (VSDs), endocardial cushion defects, and some single-ventricle defects. Congenital AV block in the setting of a structurally normal heart has been seen in children born to mothers with SLE. Iatrogenic AV block may occur during mitral or aortic valve surgery, rarely in the setting of thoracic radiation, and as a consequence of catheter ablation. AV block is a decidedly rare complication of the surgical repair of VSDs or ASDs but may complicate repairs of transposition of the great arteries.
Coronary artery disease may produce transient or persistent AV block. In the setting of coronary spasm, ischemia, particularly in the right coronary artery distribution, may produce transient AV block. In acute myocardial infarction (MI), AV block transiently develops in 10–25% of patients; most commonly, this is first-or second-degree AV block, but complete heart block (CHB) may also occur. Second-degree and higher-grade AV block tends to occur more often in inferior than in anterior acute MI; however, the level of block in inferior MI tends to be in the AV node with more stable, narrow escape rhythms. In contrast, acute anterior MI is associated with block in the distal AV nodal complex, His bundle, or bundle branches and results in wide complex, unstable escape rhythms and a worse prognosis with high mortality rates.
ELECTROCARDIOGRAPHY AND ELECTROPHYSIOLOGY OF AV CONDUCTION BLOCK
AV conduction block typically is diagnosed electrocardiographically, which characterizes the severity of the conduction disturbance and allows one to draw inferences about the location of the block. AV conduction block manifests as slow conduction in its mildest forms and failure to conduct, either intermittent or persistently, in more severe varieties. First-degree AV block (PR interval >200 ms) is a slowing of conduction through the AV junction (Fig. 275-1). The site of delay is typically in the AV node but may be in the atria, bundle of His, or His-Purkinje system. A wide QRS is suggestive of delay in the distal conduction system, whereas a narrow QRS suggests delay in the AV node proper or, less commonly, in the bundle of His. In second-degree AV block there is an intermittent failure of electrical impulse conduction from atrium to ventricle. Second-degree AV block is subclassified as Mobitz type I (Wenckebach) or Mobitz type II. The periodic failure of conduction in Mobitz type I block is characterized by a progressively lengthening PR interval, shortening of the RR interval, and a pause that is less than two times the immediately preceding RR interval on the electrocardiogram (ECG). The ECG complex after the pause exhibits a shorter PR interval than that immediately preceding the pause (Fig. 275-2). This ECG pattern most often arises because of decremental conduction of electrical impulses in the AV node.
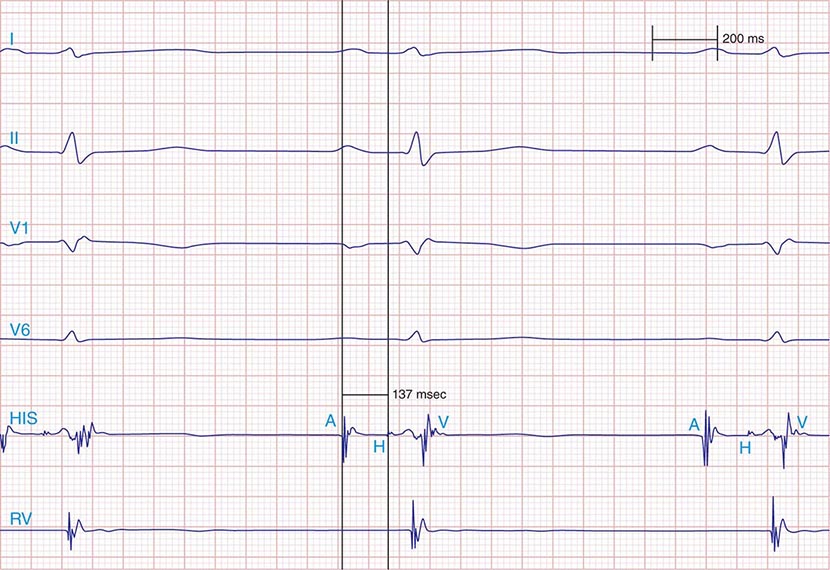
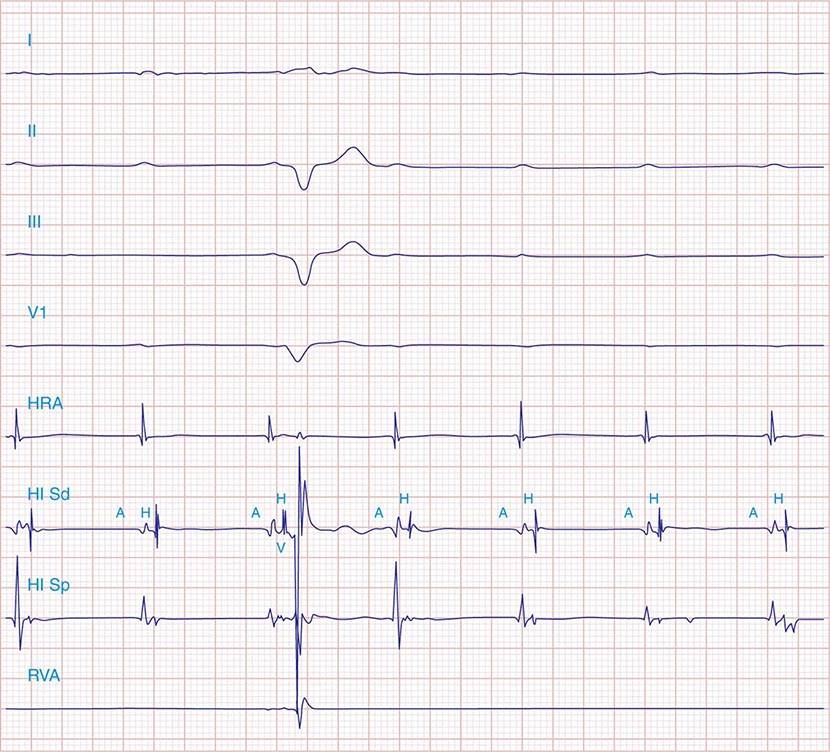
FIGURE 275-1 First-degree AV block with slowing of conduction in the AV node as indicated by the prolonged atrial-to-His bundle electrogram (AH) interval, in this case 157 ms. The His bundle-to-earliest ventricular activation on the surface ECG (HV) interval is normal. The normal HV interval suggests normal conduction below the AV node to the ventricle. I and V1 are surface ECG leads, and HIS is the recording of the endocavitary electrogram at the His bundle position. A, H, and V are labels for the atrial, His bundle, and right ventricular electrograms, respectively.
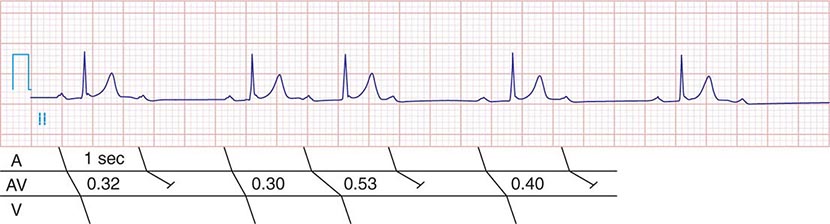
FIGURE 275-2 Mobitz type I second-degree AV block. The PR interval prolongs before the pause, as shown in the ladder diagram. The ECG pattern results from slowing of conduction in the AV node.
It is important to distinguish type I from type II second-degree AV nodal block because the latter has more serious prognostic implications. Type II second-degree AV block is characterized by intermittent failure of conduction of the P wave without changes in the preceding PR or RR intervals. When AV block is 2:1, it may be difficult to distinguish type I from type II block. Type II second-degree AV block typically occurs in the distal or infra-His conduction system, is often associated with intraventricular conduction delays (e.g., bundle branch block), and is more likely to proceed to higher grades of AV block than is type I second-degree AV block. Second-degree AV block (particularly type II) may be associated with a series of nonconducted P waves, referred to as paroxysmal AV block (Fig. 275-3), and implies significant conduction system disease and is an indication for permanent pacing. Complete failure of conduction from atrium to ventricle is referred to as complete or third-degree AV block. AV block that is intermediate between second degree and third degree is referred to as high-grade AV block and, as with CHB, implies advanced AV conduction system disease. In both cases, the block is most often distal to the AV node, and the duration of the QRS complex can be helpful in determining the level of the block. In the absence of a preexisting bundle branch block, a wide QRS escape rhythm (Fig. 275-4B) implies a block in the distal His or bundle branches; in contrast, a narrow QRS rhythm implies a block in the AV node or proximal His and an escape rhythm originating in the AV junction (Fig. 275-4A). Narrow QRS escape rhythms are typically faster and more stable than wide QRS escape rhythms and originate more proximally in the AV conduction system.
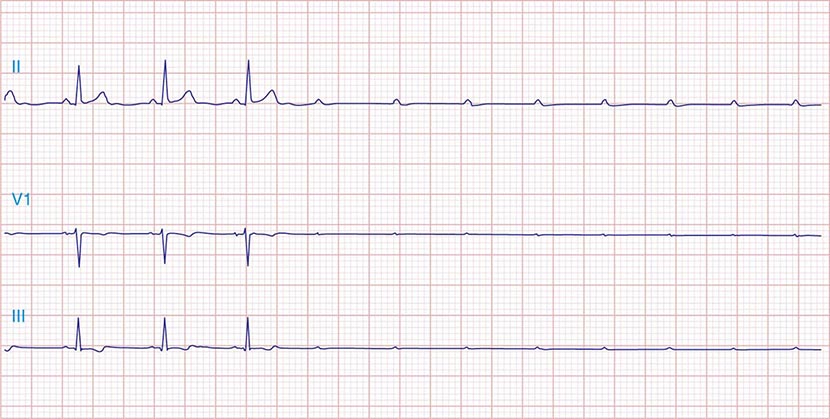
FIGURE 275-3 Paroxysmal AV block. Multiple nonconducted P waves after a period of sinus bradycardia with a normal PR interval. This implies significant conduction system disease, requiring permanent pacemaker implantation.
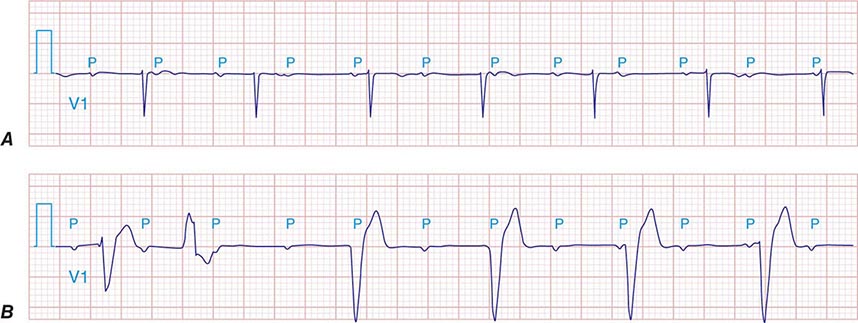
FIGURE 275-4 High-grade AV block. A. Multiple nonconducted P waves with a regular narrow complex QRS escape rhythm probably emanating from the AV junction. B. A wide complex QRS escape and a single premature ventricular contraction. In both cases, there is no consistent temporal relationship between the P waves and QRS complexes.
DIAGNOSTIC TESTING
Diagnostic testing in the evaluation of AV block is aimed at determining the level of conduction block, particularly in asymptomatic patients, since the prognosis and therapy depend on whether the block is in or below the AV node. Vagal maneuvers, carotid sinus massage, exercise, and administration of drugs such as atropine and isoproterenol may be diagnostically informative. Owing to the differences in the innervation of the AV node and infranodal conduction system, vagal stimulation and carotid sinus massage slow conduction in the AV node but have less of an effect on infranodal tissue and may even improve conduction due to a reduced rate of activation of distal tissues. Conversely, atropine, isoproterenol, and exercise improve conduction through the AV node and impair infranodal conduction. In patients with congenital CHB and a narrow QRS complex, exercise typically increases heart rate; by contrast, those with acquired CHB, particularly with wide QRS, do not respond to exercise with an increase in heart rate.
Additional diagnostic evaluation, including electrophysiologic testing, may be indicated in patients with syncope and suspected high-grade AV block. This is particularly relevant if noninvasive testing does not reveal the cause of syncope or if the patient has structural heart disease with ventricular tachyarrhythmias as a cause of symptoms. Electrophysiologic testing provides more precise information regarding the location of AV conduction block and permits studies of AV conduction under conditions of pharmacologic stress and exercise. Recording of the His bundle electrogram by a catheter positioned at the superior margin of the tricuspid valve annulus provides information about conduction at all levels of the AV conduction axis. A properly recorded His bundle electrogram reveals local atrial activity, the His electrogram, and local ventricular activation; when it is monitored simultaneously with recorded body surface electrocardiographic traces, intraatrial, AV nodal, and infranodal conduction times can be assessed (Fig. 275-1). The time from the most rapid deflection of the atrial electrogram in the His bundle recording to the His electrogram (AH interval) represents conduction through the AV node and is normally <130 ms. The time from the His electrogram to the earliest onset of the QRS on the surface ECG (HV interval) represents the conduction time through the His-Purkinje system and is normally ≤55 ms.
Rate stress produced by pacing can unveil abnormal AV conduction. Mobitz I second-degree AV block at short atrial paced cycle lengths is a normal response. However, when it occurs at atrial cycle lengths >500 ms (<120 beats/min) in the absence of high vagal tone, it is abnormal. Typically, type I second-degree AV block is associated with prolongation of the AH interval, representing conduction slowing and block in the AV node. AH prolongation occasionally is due to the effect of drugs (beta blockers, calcium channel blockers, digitalis) or increased vagal tone. Atropine can be used to reverse high vagal tone; however, if AH prolongation and AV block at long pacing cycle lengths persists, intrinsic AV node disease is likely. Type II second-degree block is typically infranodal, often in the His-Purkinje system. Block below the node with prolongation of the HV interval or a His bundle electrogram with no ventricular activation (Fig. 275-5) is abnormal unless it is elicited at fast pacing rates or short coupling intervals with extra stimulation. It is often difficult to determine the type of second-degree AV block when 2:1 conduction is present; however, the finding of a His bundle electrogram after every atrial electrogram indicates that block is occurring in the distal conduction system.
FIGURE 275-5 High-grade AV block below the His. The AH interval is normal and is not changing before the block. Atrial and His bundle electrograms are recorded consistent with block below the distal AV junction. I, II, III, and V1 are surface ECG leads. HISp, HISd, and RVA are the proximal HIS, distal HIS, and right ventricular apical electrical recordings, respectively. A, H, and V represent the atrial, His, and ventricular electrograms on the His bundle recording, respectively. (Tracing courtesy of Dr. Joseph Marine; with permission.)
Intracardiac recording at electrophysiologic study that reveals prolongation of conduction through the His-Purkinje system (i.e., long HV interval) is associated with an increased risk of progression to higher grades of block and is generally an indication for pacing. In the setting of bundle branch block, the HV interval may reveal the condition of the unblocked bundle and the prognosis for developing more advanced AV conduction block. Prolongation of the HV interval in patients with asymptomatic bundle branch block is associated with an increased risk of developing higher-grade AV block. The risk increases with greater prolongation of the HV interval such that in patients with an HV interval >100 ms, the annual incidence of complete AV block approaches 10%, indicating a need for pacing. In patients with acquired CHB, even if intermittent, there is little role for electrophysiologic testing, and pacemaker implantation is almost always indicated.
276 | Supraventricular Tachyarrhythmias |
Supraventricular tachyarrhythmias originate from or are dependent on conduction through the atrium or atrioventricular (AV) node to the ventricles. Most produce narrow QRS-complex tachycardia (QRS duration <120 ms) characteristic of ventricular activation over the Purkinje system. Conduction block in the left or right bundle branch or activation of the ventricles from an accessory pathway produces a wide QRS complex during supraventricular tachycardia that must be distinguished from ventricular tachycardia (Chap. 277). Supraventricular tachyarrhythmia may be divided into physiologic sinus tachycardia and pathologic tachycardia (Table 276-1). The prognosis and treatment vary considerably depending on the mechanism and underlying heart disease.
SUPRAVENTRICULAR TACHYCARDIA |
Abbreviations: AV, atrioventricular; VT, ventricular tachycardia.
Supraventricular tachycardia can be of brief duration, termed nonsustained, or can be sustained such that an intervention, such as cardioversion or drug administration, is required for termination. Episodes that occur with sudden onset and termination are referred to as paroxysmal. Paroxysmal supraventricular tachycardia (PSVT) refers to a family of tachycardias including AV node reentry, AV reentry using an accessory pathway, and atrial tachycardia.
CLINICAL PRESENTATION
Symptoms of supraventricular arrhythmia vary depending on the rate, duration, associated heart disease, and comorbidities and include palpitations, chest pain, dyspnea, diminished exertional capacity, and occasionally syncope. Rarely, a supraventricular arrhythmia precipitates cardiac arrest in patients with the Wolff-Parkinson-White syndrome or severe heart disease, such as hypertrophic cardiomyopathy.
Diagnosis requires obtaining an electrocardiogram (ECG) at the time of symptoms. For transient arrhythmia, ambulatory ECG recording is warranted (see Table 277-1). Exercise testing is useful for assessing exercise-related symptoms. Occasionally an invasive electrophysiology study is warranted to provoke the arrhythmia with pacing, confirm the mechanism, and often, perform catheter ablation.
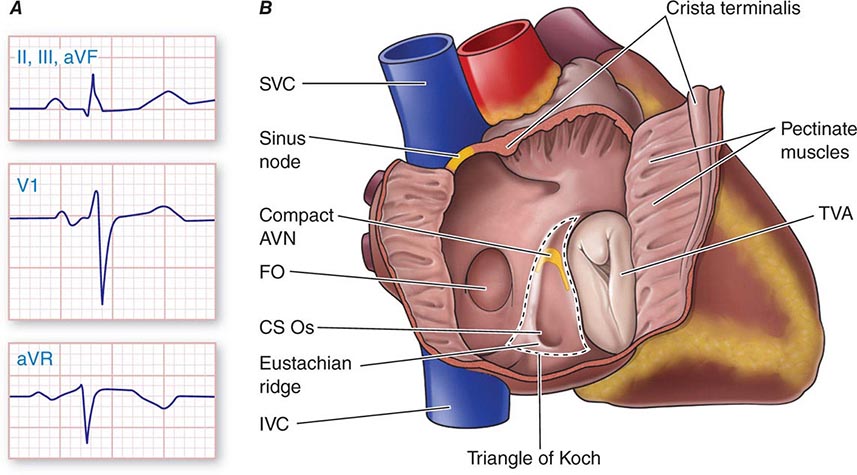
Physiologic Sinus Tachycardia The sinus node is comprised of a group of cells dispersed within the superior aspect of the thick ridge of muscle known as the crista terminalis where the posterior smooth atrial wall derived from the sinus venosus meets the trabeculated anterior portion of the right atrium (Fig. 276-1). Sinus p waves are characterized by a frontal plane axis directed inferiorly and leftward, with positive p waves in leads II, III, and aVF; a negative p wave in aVR; and an initially positive biphasic p wave in V1. Normal sinus rhythm has a rate of 60–100 beats/min. Sinus tachycardia (>100 beats/min) typically occurs in response to sympathetic stimulation and vagal withdrawal, whereby the rate of spontaneous depolarization of the sinus node increases and the focus of earliest activation within the node typically shifts more leftward and closer to the superior septal aspect of the crista terminalis, thus producing taller p waves in the inferior limb leads when compared to normal sinus rhythm.
FIGURE 276-1 Right atrial anatomy pertinent to normal sinus rhythm and supraventricular tachycardia. A. Typical P-wave morphology during normal sinus rhythm based on standard 12-lead electrocardiogram. There is a positive P wave in leads II, III, and aVF; biphasic, initially positive P wave in V1; and negative P wave in aVR. B. Right atrial anatomy seen from a right lateral perspective with the lateral wall opened to view the septum. AVN, atrioventricular node; CS Os, coronary sinus ostium; FO, fossa ovalis; IVC, inferior vena cava; SVC, superior vena cava; TVA, tricuspid valve annulus.
Sinus tachycardia is considered physiologic when it is an appropriate response to exercise, stress, or illness. Sinus tachycardia can be difficult to distinguish from focal atrial tachycardia (see below) that originates from a focus near the sinus node. A causative factor (such as exertion) and a gradual increase and decrease in rate favors sinus tachycardia, whereas an abrupt onset and offset favor atrial tachycardia. The distinction can be difficult and occasionally requires extended ECG monitoring or even invasive electrophysiology study. Treatment for physiologic sinus tachycardia is aimed at the underlying condition (Table 276-2).
COMMON CAUSES OF PHYSIOLOGIC SINUS TACHYCARDIA |