The Basic Structure of the Mammalian Cell
A cell is a self-contained fundamental unit of life. All cells are tridimensional, space-occupying structures, although when spread on a glass slide and viewed through the light microscope, they appear to be flat. Each mammalian cell has three essential components: cell membrane, cytoplasm, and nucleus (Fig. 2-1 and see Frontispiece and Fig. 3-1). The cell membrane encloses the transparent cytoplasm. Within the cytoplasm, enclosed in its own membrane or envelope, there is a smaller, approximately spherical dense structure—the nucleus. The nucleus is the principal repository of deoxyribonucleic acid (DNA), the molecule governing the genetic and functional aspects of cell activity (see Chap. 3). Although some mammalian cells, such as erythrocytes or squamous cells, may lose their nucleus in the final stages of their life cycle, even these final events are programmed by their DNA. All nucleated cells are classified as eukaryotic cells (from Greek, karion = kernel, nucleus) in contrast with primitive cells, such as bacteria, wherein the DNA is present in the cytoplasm but is not enclosed by a membrane as a distinct nuclear structure (prokaryotic cells). Many of the fundamental discoveries pertaining to the molecular biology of cells were made in prokaryotic cells, documenting that all basic biochemical manifestations of life have a common origin. Families of cells differ from each other by their structural features (morphology) and by their activities, all programmed by DNA. The recognition of these cell types and their alterations in health and disease is the principal task of diagnostic cytology. All cells share the fundamental structural components that will be described in these pages.
MICROSCOPIC TECHNIQUES USED IN EXAMINATION OF CELLS
Cells can be examined by a variety of techniques, ranging from the commonly used light and electron microscopy to newer techniques of confocal and digital microscopy. Additional information on cell structure, derivation, and function can be obtained by immunocytochemistry and by in situ hybridization of cell components. The techniques required for special procedures will be described in the appropriate chapters. This brief summary will serve as an introduction to the description of the fundamental structure of the cell.
Light Microscopy
Bright-Field Light Microscopy
Bright-field light microscopes are optical instruments that allow the examination of cells at magnifications varying from 1× to 2,000×, using an appropriate combination of lenses. The highest resolution of the commonly used light microscopes, that is, the ability of the instruments to visualize the smallest objects, is limited by the wavelength of the visible spectrum of light, which is about 0.5 μm. The principles of bright-field light microscopy have been described in numerous books and manuals and need not be repeated here. It is assumed that the readers have a working knowledge of these instruments. Suffice it to say that the quality of the optics used, skill in the adjustment of the illumination, and the depth of the microscope’s focus are essential
in evaluating the cellular preparations. In practice of clinical cytology, bright-field microscopy satisfies nearly all requirements for the diagnostic assessment of cells. The same technique is used in assessing the results of special stains and of immunocytochemistry.
in evaluating the cellular preparations. In practice of clinical cytology, bright-field microscopy satisfies nearly all requirements for the diagnostic assessment of cells. The same technique is used in assessing the results of special stains and of immunocytochemistry.
Preparation of Cells for Bright-Field Light Microscopic Examination
The cells are usually prepared for a light microscopic examination in the form of direct smears on commercially available glass slides of predetermined thickness and optical quality. Samples of cells suspended in fluid may be placed on glass slides by means of a special centrifuge, known as a cytocentrifuge, or a similar apparatus. A cell suspension may also be filtered across a porous membrane. The cells deposited on the surface of such membranes may either be examined directly or may be placed on glass slides by a process of reverse filtration. Cell samples may also be studied in histologic-type sections, after embedding of the sediment in paraffin (a technique known as the cell block). For details of these techniques, see Chapter 44.
Fixation.
Fixation of cell preparations is a common procedure having for its purpose the best possible preservation of cell components after removal from the tissue of origin. A variety of fixatives may serve this purpose, all described in Chapter 44. However, diagnostic techniques may also be based on air-dried cell preparations, either unfixed or postfixed in methanol, which introduce a number of useful artifacts. Such techniques are used in hematology and in aspiration biopsy samples.
Staining.
Optimal results in bright-field microscopy are obtained on stained preparations that provide visible contrast and discrimination among the cell components. A variety of stains, described in Chapter 44, can be used to best demonstrate various cell components. Common stain combinations use hematoxylin and its variants as the nuclear stain and eosin or its many variants as the cytoplasmic stain. Examples of stains of this type include the hematoxylin-eosin stain and the Papanicolaou stain, which allow for a good visualization of the principal components of the cell, by contrasting the nucleus and the cytoplasm. Other stains in common use include methylene blue, toluidine blue, and Giemsa colorant that provide less contrast among cell components but have the advantage of rapidity of use. An example of cells fixed in alcohol and stained by the Papanicolaou method is shown in Figure 2-4.
Phase-Contrast Microscopy
Phase-contrast microscopy utilizes the difference in light diffraction among the various cell components and special optics that allow the visualization of components of unstained cells. The Nomarski technique is a variant of phase contrast microscopy that is particularly useful in the study of cell surfaces. Either technique may be applied to the study of living cells in suspension or culture and, when coupled with time-lapse cinematography or a television system, may
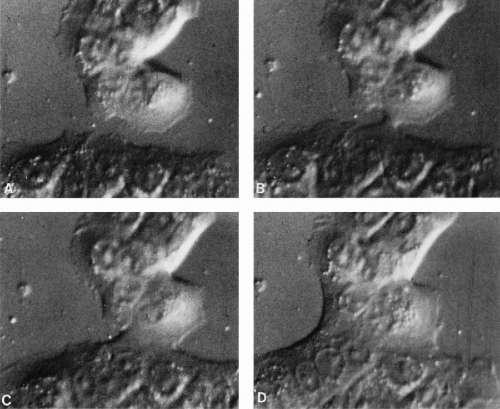
provide a continuous record of cell movements and behavior. These techniques are particularly useful in experimental systems, as they may document the differences in cell behavior under various circumstances, for example, after treatment of cultured cells with a drug or during a genetic manipulation. The systems also allow the study of events, such as movement of chromosomes during cell division, or mitosis. An example of the application of the Nomarski technique to a cell culture is shown in Figure 2-2 .
provide a continuous record of cell movements and behavior. These techniques are particularly useful in experimental systems, as they may document the differences in cell behavior under various circumstances, for example, after treatment of cultured cells with a drug or during a genetic manipulation. The systems also allow the study of events, such as movement of chromosomes during cell division, or mitosis. An example of the application of the Nomarski technique to a cell culture is shown in Figure 2-2 .
Fluorescent Microscopy
Cells or cell components stained with fluorescent compounds or probes can be visualized with the help of microscopes provided with special lenses and a source of fluorescent light, such as a mercury bulb or a laser, tuned to an appropriate wavelength, exciting fluorescence of the probe. In highly specialized commercial systems, the amount of fluorescence can be measured in individual cells or families of cells, and may serve to quantify various cell components. A somewhat similar system is used in flow cytometry (see Chap. 47). Fluorescence microscopy is particularly valuable in the procedures known as in situ hybridization, with the purpose of documenting the presence of chromosomes, chromosomal aberrations, or individual genes (see Fig. 2-31 and Figs. 4-26, 4-27, and 4-29). Fluorescent microscopy is also useful in identifying certain components of cell cytoplasms or cell membranes, using specific antibodies. Application of fluorescent microscopy and other techniques to the study of living cells was summarized in a series of articles on biologic imaging in the journal, Science, 2003.
Confocal Microscopy
Using a system of complex optics and a laser, the technique, combined with phase and fluorescent microscopy in complex and costly instruments, allows the visualization of cells and tissues in slices, separated from each other by approximately 1 μm. The images of the slices can be combined on a computer to give a three-dimensional picture of the cell or tissue and their components. This technique is applicable to individual cells or cell clusters that can be examined layer-by-layer.
Digital Microscopy
With the wide availability of sophisticated computers, it has become possible to transform cell images into digits, that is, numerical values. The images are recorded by television or digital cameras, transformed into numerical values and stored in the computers’ memory, on videotape, or on a videodisc. The original images can be reconstituted when needed. Such images, often of outstanding quality, can be manipulated with the help of special software. Images from several different sources can be assembled into plates suitable for publications or special displays. The colors of the displays can be adjusted for optimal quality of images. Many new plates in this book have been prepared with this technique. Digital microscopy can also be applied to electron microscopic images (Shotton, 1995).
Digital microscopy has been extensively applied in analytical and quantitative studies of cells and cell components. These techniques allow discrimination among families of cells of similar appearance but different biologic or clinical significance. They can also be applied to a variety of measurements of cell components, such as DNA, as discussed in Chapter 46. Variants of these techniques have been used in commercial instruments for automated or semiautomated analysis of cell populations.
Digital microscopy is suitable for direct transmission of images via cable or satellites to remote locations (telepathology or telecytology) for teaching or diagnostic purposes, as discussed in Chapters 1 and 46. Demonstration projects of this technology have documented that such images are of good quality when examined at the receiving stations. The images can be studied under variable magnification factors, thus allowing for diagnostic opinions. Transmissions of images by Internet have been extensively used for teaching. It is conceivable that, in the future, central telepathology consultation centers will be established to advise pathologists on difficult cases. At present, the systems are limited by cost, the speed of transmission, and by the availability of knowledgeable consultants to perform such services.
Electron Microscopy
Transmission Electron Microscopy
Transmission electron microscopic technique utilizes certain optical properties of a fixed beam of electrons to illuminate the object. The images are captured on photographic plates. Extremely thin sections of tissues or cells (50 to 100 nm) and staining with heavy metals are required. Special fixation and embedding techniques must be used. The method allows a unique insight into the fine structure of the cell. Most of the images in this chapter were obtained by this technique.
Scanning Electron Microscopy
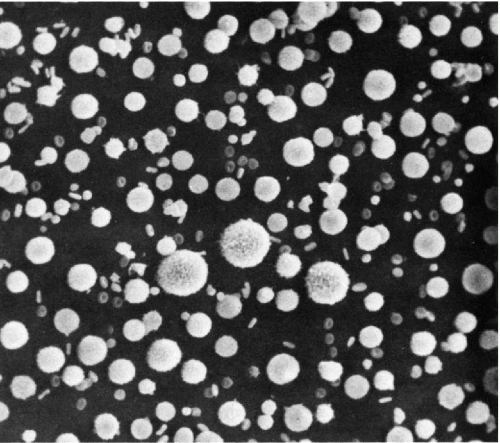
In the commonly used mode, the scanning electron microscopy technique utilizes a rapidly moving beam of electrons to scan the surface of cells or other objects. The cells are dehydrated, fixed, and coated with a thin metallic layer, usually of gold and palladium. The metal forms an exact replica of the cell surface. The beam of electrons glides over the metallic surface, and the reflected electrons form an image that may be registered on a photographic plate (Fig. 2-3) or on a fluorescent screen. Scanning electron microscopy is also applicable to the freeze-fracture technique, described below.
Other Techniques
Several other special techniques, such as interference microscopy and x-ray diffraction microscopy, have been used for a variety of investigative purposes. Scanning-tunneling microscopy is a new tool for visualization of surfaces of molecules such as DNA. This technique has no applications to diagnostic cytology.
Magnetic resonance, a technique widely used in imaging of the human body (MRI), is applicable to the study of tissues in vitro and to histologic sections as magnetic resonance microscopy (Huesgen et al, 1993; Sbarbati and Osculati, 1996; Johnson et al, 1997). The technique is based on magnetic gradients that produce a shift in hydrogen ions’ alignment in water content of the living tissues, creating images that can be captured by computer and recorded on a photographic plate. Because of its low resolution, the practical value of this technique remains to be determined.
THE COMPONENTS OF THE CELL
The components of the cell will be described under three main headings: the cell membrane, the cytoplasm, and the nucleus (see Frontispiece). Whenever possible, the description will comprise light and electron microscopic
observations. The purely morphologic description has limited bearing on the intimate biochemical interrelationship of the cell components. The reader is referred to Chapter 3 and the appended references for further information.
observations. The purely morphologic description has limited bearing on the intimate biochemical interrelationship of the cell components. The reader is referred to Chapter 3 and the appended references for further information.
The Cell Membrane
The cell membrane is the outer boundary of the cell, facilitating and limiting the exchange of substances between the cell and its environment. In light microscopy, the membrane of well-fixed mammalian cells cannot be seen. The cell’s periphery appears as a thin condensation (Fig. 2-4).
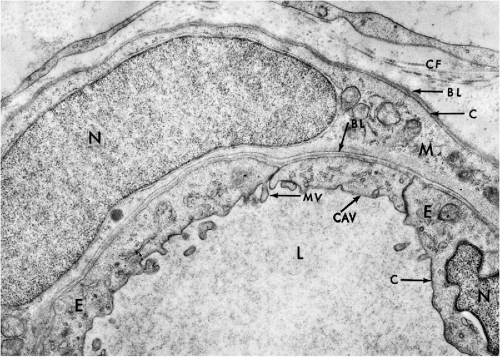
In transmission electron microscopy, the cell membrane appears as a well-defined line measuring approximately 75 Å in width (Fig. 2-5). The membrane is composed of three layers, each about 25 Å thick (see Frontispiece and Fig. 2-18). The inner and the outer dense (electronopaque) layers are separated by a somewhat wider lucent central layer. Similarly constructed membrane systems are observed in a variety of intracytoplasmic components within the cell, such as the mitochondria and the endoplasmic reticulum (see below). The term unit membrane is often used in reference to cell membranes in general.
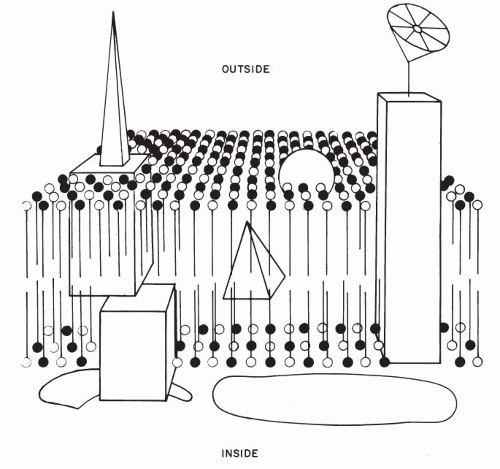
Davson and Danielli (1952) proposed that the plasma membrane is composed of a double lipid layer coated by polypeptide chains of protein molecules. This concept was acceptable so long as it readily explained certain physicochemical characteristics (semipermeability) of cell membranes. However, it has become evident that the cell membrane, far from being a passive envelope of cell contents, plays a critical role in virtually every aspect of cell function. Thus, the cell membrane regulates the internal environment of the cell, participates actively in recognition of the external environment and in transport of substances to and from the cell, determines the immunologic makeup of the cells, and accounts for the interrelationship of cells.
The initial insight into the makeup and function of the cell membrane was based on the study of erythrocytes. Their membrane is made up of a double layer (bilayer) of lipids, formed by molecules provided with chains of fatty acids. The lipid molecules have one water-soluble (or hydrophilic) end and a water-insoluble (or hydrophobic) end. In the cell membrane, the electrically charged hydrophilic ends of the lipid molecules form the inner and the outer surfaces of the cell membrane, whereas the uncharged, hydrophobic chains of fatty acids are directed toward the center of the cell membrane, away from the two surfaces. Cholesterol molecules add structural rigidity to the cell membrane. Protein molecules of various sizes, functions, and configurations are located within the lipid bilayer (integral proteins) but also extend beyond the cell membrane, either to the outside or to the inside of the cell or both. Such transmembrane proteins provide communication between the cell environment and cell interior. The number, makeup, position, and mobility of the protein molecules account for specific, individual properties of cells and tissues by forming specific receptor molecules. Cell membranes are further characterized by molecules of carbohydrates that attach either to the lipids (glycolipids) or to the proteins (glycoproteins) and which are the repository of the immunologic characteristics of the cell.
On the inner (cytoplasmic) aspect of the cell membrane, other protein molecules have been identified (peripheral proteins). Their function appears to be structural in maintaining the integrity of the cell membrane and in providing communication between the cell membrane and the interior of the cell (Fig. 2-6).
This complex asymmetric structure of the cell membrane cannot be demonstrated by conventional electron microscopy.
Therefore, to study the problem, special techniques have been applied, such as freeze-fracture. The freeze-fracture technique consists of three steps: very rapid freezing of cells and tissues, fracturing the tissue with an instrument, and preparation of a metal replica of the fractured surface that can be examined in the scanning electron microscope. It has been determined that the fracture lines are not distributed in a haphazard fashion but, rather, run along certain predetermined planes.
Therefore, to study the problem, special techniques have been applied, such as freeze-fracture. The freeze-fracture technique consists of three steps: very rapid freezing of cells and tissues, fracturing the tissue with an instrument, and preparation of a metal replica of the fractured surface that can be examined in the scanning electron microscope. It has been determined that the fracture lines are not distributed in a haphazard fashion but, rather, run along certain predetermined planes.
Freeze-fracture of cell membranes disclosed two surfaces that, by agreement, have been named the P face and E face (Fig. 2-7). The P face represents the inner aspect of the cell membrane and contains numerous protruding protein particles. The E face represents the outer part of the cell membrane, which is relatively smooth, except for pits corresponding to the protein particles attached to the P face. A few protein particles usually remain attached to the E face. The density and distribution of the protein particles varies from cell type to cell type and may be substantially modified by immunologic and chemical methods, indicating that the position of these particles within the cell membrane is not fixed. Thus, the cell membrane is now thought to be a fluid-mosaic membrane, as first proposed by Singer and Nicholson (1972). It may be conceived as a viscous structure that can adapt itself to changing needs and conditions by being permissive to movements of large molecules, such as protein particles. Fixation of cells solidifies the membrane. The freeze-fracture images represent only snapshots of the position of the protein particles at the time of fixation.
The freeze-fracture technique may also be used to study the structure of cell junctions (see Fig. 2-16) and the interior of other cell membranes, such as the nuclear envelope (see Fig. 2-27).
The basic structure of intracellular membranes, such as those composing the endoplasmic reticulum or mitochondria, appears to be essentially similar to that of the cell membrane, but differs in lipid/protein ratios and associated proteins and enzymes, reflecting the diversity of functions.
Cytoplasmic Interactions
Extensive work has been performed in recent years to establish links between the cell membrane and the cytoplasm. It is quite evident that this must be a very intimate association, as cell function depends on signals and nutrients received through the cell membrane. Also, the export of substances manufactured by the cell (or products of cell metabolism) must be regulated by interaction between the cytoplasm and the cell membrane.
Molecular biologic investigations of recent years have identified numerous protein molecules that contribute to the function of the cell membrane as a flexible link between the environment and the interior of the cell. Each one of these molecules interacts with other molecules and theseinteractions are growing increasingly complex. So far, only
small fragments of this knowledge have emerged. At thetime of this writing (2004), no clear, cohesive picture has been formulated to explain how the cell membrane functions. Suffice it to say that there is good evidence that the cell membrane plays an important role in virtually every aspect of cell function in health and disease. Luna and Hitt (1992) discussed the interaction between the cell skeleton and cell membrane as one example of these interactions. Among the components of the cell skeleton that interact with the cell membrane are the intermediate filaments and tubules, described further on in this chapter.
small fragments of this knowledge have emerged. At thetime of this writing (2004), no clear, cohesive picture has been formulated to explain how the cell membrane functions. Suffice it to say that there is good evidence that the cell membrane plays an important role in virtually every aspect of cell function in health and disease. Luna and Hitt (1992) discussed the interaction between the cell skeleton and cell membrane as one example of these interactions. Among the components of the cell skeleton that interact with the cell membrane are the intermediate filaments and tubules, described further on in this chapter.
The cell membrane is also the site of molecules that define the immunologic features of the cell. For example, the clusters of differentiation (CD) and blood group antigens discussed elsewhere in this book, are located on the cell membranes.
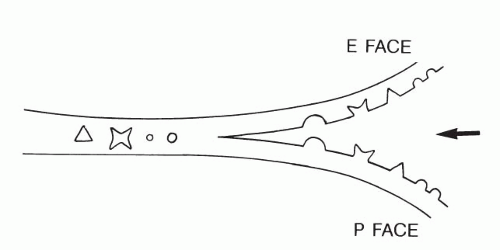 Figure 2-7 Principle of freeze-fracture. The sharp wedge (arrow) separates the frozen membrane into two faces (P and E; see text) without disturbing the position of intermembrane protein particles or structures (see Figs. 2-16 and 2-27). |
Coated Pits, Vesicles, and Caveolae: Mechanisms of Import and Export
Import, export, and transport of a variety of molecules within the cytoplasm takes place through pits and vesicles formed by invagination of cellular membranes. The largest of such vesicles observed on cell surfaces are known as pinocytotic vesicles. The pits and vesicles are coated by molecules of a complex protein, clathrin, which appears to be present in all cells. Clathrin is composed of three heavy and three light protein chains that form the scaffolds of the coats. Clathrin requires the cooperation of other proteins known as adaptors to fulfill its many functions, which include capturing, sorting, and transporting molecules. The molecular mechanisms of endocytosis have been extensively studied (Gillooly and Stemark, 2001). It may be assumed that each pit or vesicle is provided with specific receptors to a molecule or molecules of importance to the cell, and that it will recognize and selectively capture this molecule or molecules from thousands of molecules circulating within the fluid bathing the cell. Once the selected substance is captured, the vesicle closes and sinks into the cytoplasm to deliver its cargo to its appropriate destination. However, nature is extremely economical, and there is excellent evidence that the fragment of cell membrane that is used to form a vesicle is recirculated and returned to the surface in a different location to serve again. A similar mechanism is observed in removal or phagocytosis of hostile substances (or organisms, such as bacteria) that are recognized by the receptors on the cell surface. Removal of accumulated extracellular debris is another phagocytic function usually performed by specialized cells (macrophages) in a similar manner (see Fig. 5-13). A number of genetic disorders are now thought to be associated with defective mechanisms of intracellular membrane transport (Olkkonen and Ikonen, 2000).
A reverse mechanism occurs in export of molecules, which are packaged into vesicles formed within the cell (mainly in the Golgi apparatus) (see below) and travel to the surface. The vesicles attach to the inner aspect of the cell membrane by means of specific receptors. After the merger, the cell membrane splits open, and the content of the vesicles is discharged into the circulating fluid bathing the cell.
Besides clathrin-coated pits, the cell membrane also forms specific small invaginations (50 to 100 nm in diameter) that are known as caveolae. In cross-section, the caveolae appear as small, spherical vesicles in the adjacent cytoplasm (see Fig. 2-5). They are particularly prevalent in endothelial cells, smooth muscle cells, and type I pneumocytes (Schlegel et al, 1998; Couvet et al, 1997). The caveolae are composed of caveolins, a family of integrated membrane proteins, which interact with a number of signaling molecules and thus regulate the cell’s responses to its environment (Okamoto et al, 1998). Thus, caveolins have been implicated in cells’ response to injury and may play a role in human breast cancer (Engelman, 1998).
Specialized Structures of Cell Surfaces
Transmission electron microscopy has been helpful in elucidating some of the structural details of specialized structures of cell surfaces and the manner in which cells are attached to each other.
The Glycocalix
Specialized techniques of electron microscopy serve to demonstrate an ill-defined, fuzzy layer of material on the free surfaces of cells. This layer is referred to as glycocalix and appears to be composed primarily of glycoproteins containing residues of sialic acid. Although the thickness and, presumably, chemical makeup of glycocalix vary from one type of cell to another, its occurrence is a rather generalized phenomenon, the exact function of which is not well understood.
Cilia and Flagella: Motile Cell Processes
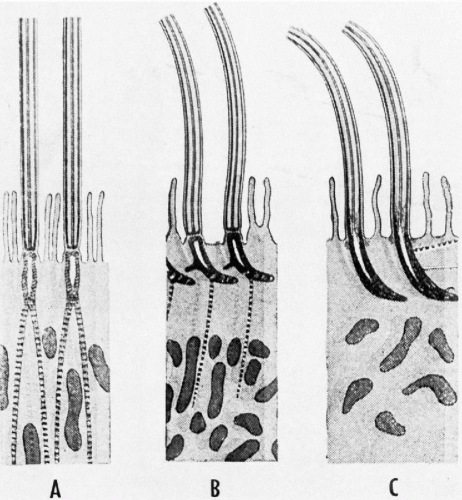
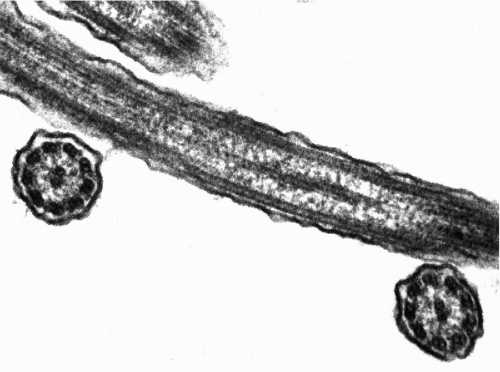
The cilia and flagella may be readily identified by light microscopy. Both are mobile extensions of the cell membrane and are capable of rapid movements. A flagellum is usually a single, elongated mobile part of the cell, as observed in spermatozoa. Cilia are shorter and multiple, usually functioning (batting) in a synchronous manner, for example, in cells lining the bronchial epithelium (see Fig. 2-4), or other epithelia, such as that of the fallopian tube and the endocervix. Cells bearing cilia are usually polarized; that is, they have a specific spatial orientation in keeping with their function: the cilia are usually oriented toward the lumen of an organ or tissue. The cilia are anchored in a thick, flat portion of the cell cytoplasm immediately adjacent to the surface, referred to as a terminal plate (see Fig. 2-4A). Careful observation reveals that the terminal plate is composed of a series of dense granules, or basal corpuscles, each belonging to a single cilium (see Figs. 2-4B and 2-8). Cilia are rare in cancer cells.
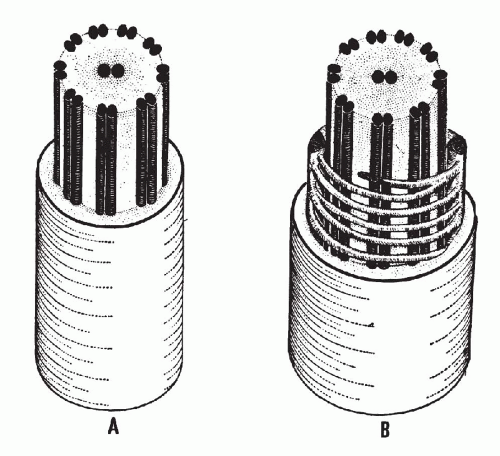
There is a remarkable uniformity of ultrastructure of the motile cell processes throughout the animal and the plant kingdoms. Each cilium or flagellum contains 11 microtubules, of which two are single and located within the center, and nine are double (doublets) and located at the periphery (Figs. 2-9 and 2-10). The structure of the cilia and flagella is very similar to that of the centrioles (see below). Species differences do exist in the manner in which the cilia and the flagella are anchored within the cytoplasm (see Fig. 2-8).
Within recent years, considerable insight has been gained into the function of the cilia and flagella. These cell processes are composed of an intricate system of protein fibrils that glide against each other in executing the movements, which require a substantial input of energy, provided by mitochondria. For details of the current concepts of movements, see Satir (1965) and Sale and Satir (1977).
Microvilli and Brush Border
Microvilli are short, slender, regular projections on free surfaces of cells that can be visualized in electron microscopy
or light microscopy. The term brush border or striated border is applied to specialized cell surfaces provided with microvilli. The brush border is observed on the free surface of the intestinal mucosa (Fig. 2-11A and see Fig. 2-15). The regular, finger-like intestinal microvilli, delimited by the plasma membrane, measure approximately 1 μm in length and serve the function of increasing the useful surface of the cell. A similarly organized brush border is observed in the proximal segment of the renal tubules. Microvilli may be observed by light microscopy on the surface of various normal human cells, as short, delicate, hair-like striations, best observed in air-dried and stained cells, spread on glass slides. Scanning electron microscopy shows microvilli, as finger-like, slender structures, projecting from the surface of the cell. Long and irregular microvilli that occur on the surfaces of cancer cells are much easier to see in light microscopy and are occasionally of diagnostic help. These observations are discussed in detail in Chapter 7 and are illustrated in Figures 7-7, 7-8, 7-9, 7-10, 7-11, 7-12, 7-13 and 7-14.
or light microscopy. The term brush border or striated border is applied to specialized cell surfaces provided with microvilli. The brush border is observed on the free surface of the intestinal mucosa (Fig. 2-11A and see Fig. 2-15). The regular, finger-like intestinal microvilli, delimited by the plasma membrane, measure approximately 1 μm in length and serve the function of increasing the useful surface of the cell. A similarly organized brush border is observed in the proximal segment of the renal tubules. Microvilli may be observed by light microscopy on the surface of various normal human cells, as short, delicate, hair-like striations, best observed in air-dried and stained cells, spread on glass slides. Scanning electron microscopy shows microvilli, as finger-like, slender structures, projecting from the surface of the cell. Long and irregular microvilli that occur on the surfaces of cancer cells are much easier to see in light microscopy and are occasionally of diagnostic help. These observations are discussed in detail in Chapter 7 and are illustrated in Figures 7-7, 7-8, 7-9, 7-10, 7-11, 7-12, 7-13 and 7-14.
Cell Contacts
The relationship of cells to one another within the same tissue or within adjoining tissues is of paramount importance for the structural integrity and function of all organs (see Fig. 2-11). These relationships are regulated by cell membranes, which form a variety of cell contacts and cell attachments. It is not known as yet whether the cell attachments are formed on predetermined specialized areas of cell surfaces, or incidental to haphazard cell contacts.
From the morphologic point of view, a number of structural cell contacts have been identified. These are the desmosomes, the junctional complexes, and the gap junctions (Fig. 2-12).
The Desmosomes and Hemidesmosomes
The structure of cell attachments, especially within the epithelia, has been of interest to biologists and pathologists alike for over a century. Early on, it has been noted in light microscopy that, within the squamous stratified epithelia, the cells are attached to each other by means of cytoplasmic extensions, named intercellular bridges. In phase microscopy, fine fibrils, named tonofibrils, may be seen converging on the areas connecting the unfixed, unstained cells. For many years, it has been known that, in the centers of the intercellular bridges, there existed small dense structures, variously referred to as granules (Ravier) or nodes (Bizzozero) and currently referred to as desmosomes. Electron microscopic studies have demonstrated that the desmosomes represent points of adhesion of two adjacent cells (see Figs. 2-11, 2-12 and 2-13). The cytoplasm of adjacent cells remains firmly attached at the points of desmosomal adherence but, owing to artifacts of
fixation, it shrinks elsewhere. The elongated desmosomebound portions of the cytoplasm constitute the intercellular bridges seen in light microscopy. Recent studies show that molecules of C-cadherin are an essential component of desmosomes (He et al, 2003).
fixation, it shrinks elsewhere. The elongated desmosomebound portions of the cytoplasm constitute the intercellular bridges seen in light microscopy. Recent studies show that molecules of C-cadherin are an essential component of desmosomes (He et al, 2003).
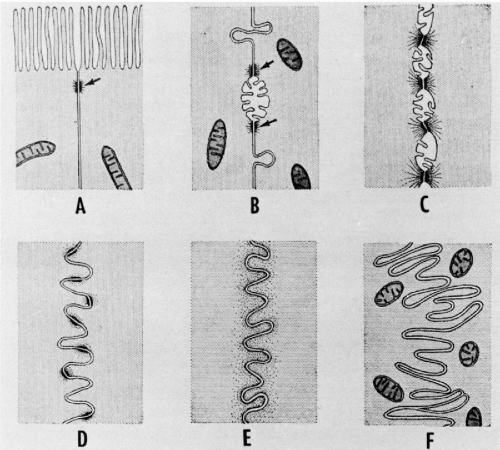 Figure 2-11 Diagrammatic representation of several types of specialization found on the surfaces of contact between adjacent cells. A. On the interface between columnar epithelial cells of the intestine, desmosomes (arrow) are frequently seen near the free surface showing striated border. B. On the contact surfaces of liver cells, desmosomes occur (arrows) on either side of the bile capillary. Near these are stud-like processes that project into concavities on the surface of the adjacent cell. C. In the stratified squamous epithelium of the rodent vagina, the cell surfaces are adherent at the desmosomes and retracted between, giving rise to the so-called intercellular bridges of light microscopy. A continuous system of intercellular spaces exists between bridges. Projecting into these spaces are a few short microvilli. D.
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|