The Anatomy of the Esophagus
Toni Lerut
Willy Coosemans
Herbert Decaluwé
Georges Decker
Paul De Leyn
Philippe Nafteux
Dirk Van Raemdonck
A the Esophagus
The esophagus is not simply a conduit to allow passage of food and drink from the mouth to the stomach. It is an intensely complex part of the gastrointestinal tract. Cranially the esophagus is a continuation of the hypopharynx and caudally it continues into the cardia of the stomach. As such, a thorough understanding of the anatomy and physiology of the esophagus should be gained before contemplating surgery of it.
Embryology
Description
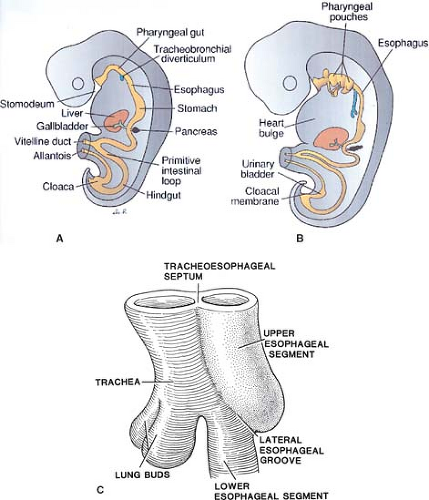
The esophagus comes from two sources of the primitive gut. The cranial portion is derived from the pharyngeal gut or pharynx and the caudal part from the pregastric segment of the foregut. With the growth of the embryo, the primitive gut lumen becomes almost filled but later, due to a process of epithelial layer vacuolization, hollows out again. At about 4 weeks of embryonic development, the laryngotracheal groove appears, subsequently forming the tracheobronchial diverticulum on the ventral surface of the foregut, at the level of the fourth pharyngeal pouches (Fig. 1A,B). The diverticulum is gradually closed by the tracheoesophageal folds (internal ridges of the lateral esophageal groove), caudally first, forming the tracheoesophageal septum (Fig. 1). The endoderm forms the mucosal epithelium and associated ducts and glands. The mesoderm forms the lamina propria, muscularis mucosa, and muscular coat, the branchial arches forming the striated muscle and the visceral splanchnic mesoderm the smooth muscle coat. Arterial and venous supply of the esophagus is segmental. The cranial arteries are derived from the branchial arches and caudal ones from branches of the aorta. With the unfolding and lengthening of the embryo, the esophagus also lengthens. The original cell lining of the esophagus changes from a two- to three-layer pseudostratified columnar epithelium via a stratified columnar stage to a stratified squamous epithelium by 90- to 130-mm embryo length.
Clinical Points of Interest
Overdevelopment of the internal ridges of the tracheoesophageal septum is proposed as the cause of esophageal atresia according
to Smith and deviation of the tracheoesophageal septum the cause of tracheoesophageal fistula. Failure of development of the tracheoesophageal septum can cause posterior laryngeal clefts. Defects of vacuolization and/or the persistence of diverticulae during the lengthening of the esophagus may be the cause of embryonic cysts, duplication cysts, webs, rings, and congenital stenosis, the latter including possibly even cartilaginous remnants or rings.
to Smith and deviation of the tracheoesophageal septum the cause of tracheoesophageal fistula. Failure of development of the tracheoesophageal septum can cause posterior laryngeal clefts. Defects of vacuolization and/or the persistence of diverticulae during the lengthening of the esophagus may be the cause of embryonic cysts, duplication cysts, webs, rings, and congenital stenosis, the latter including possibly even cartilaginous remnants or rings.
Description
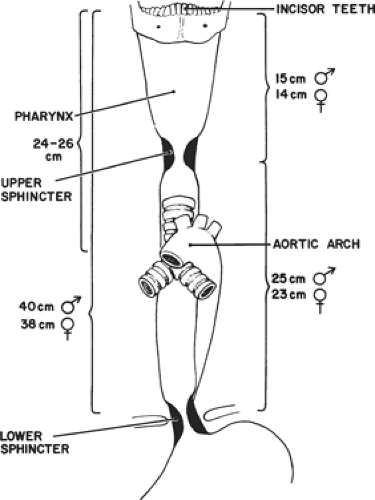
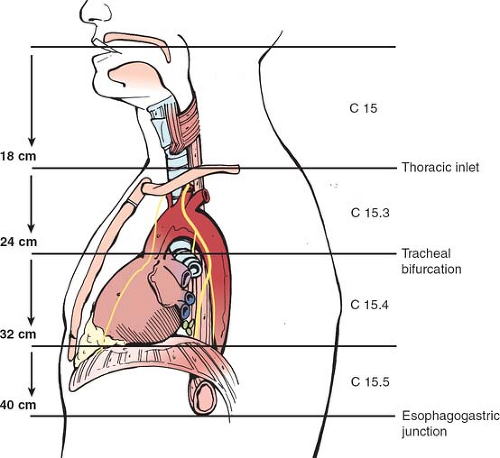
The esophagus is about 25 cm long extending from the level of the 6th cervical vertebra down to that of the 11th thoracic vertebra. It is not a fixed structure and moves cranially a few centimeters on swallowing and tilting the head backward. It is anchored superiorly to the cricoid cartilage and pharynx by strips of collagen fibers interspersed with muscle fibers. Similarly, it is tethered along its length to the left and right pleura, the aorta, the left main bronchus, and the fibrous pericardium. These are fine structures macroscopically and consist of a variable amount of collagen, elastic, and muscle fibers. It is normal clinical practice such as in endoscopy to describe the length along the esophagus as being that measured from the incisors teeth, thus including the oropharynx in this measurement. There is a narrowing at 15 to 17 cm, that is, the upper esophageal sphincter, another at about 38 to 40 cm, that is, the lower esophageal sphincter (LES), and an anterolateral indentation at about 25 cm where the left main bronchus and arch of the aorta cross it anteriorly (Fig. 2). The female’s esophagus is shorter than the male’s by a few centimeters but in both sexes there is marked variability depending on the height and weight of the individual. The esophagus shows two curves in the anteroposterior plane: although starting in the midline at the junction with the oropharynx, it deviates to the left as it descends to the thoracic inlet then returns to a midline position at about the fifth thoracic vertebra, before passing once again to the left (Fig. 3). The esophagus also follows the contour of the cervical and upper thoracic spine in a lateral plane (Fig. 4).
The latest edition of the American Joint Committee on Cancer (AJCC) cancer staging manual divides the esophagus into four parts, cervical, upper thoracic, middle thoracic, and lower thoracic esophagus/esophagogastric junction (EGJ) (Fig. 5).
The cervical esophagus lies in the neck, bordered superiorly by the hypopharynx and inferiorly by the thoracic inlet, which lies at the level of the sternal notch. Typical endoscopic measurements for the cervical esophagus measured from the incisors are from 15 to <20 cm.
The upper thoracic esophagus is bordered superiorly by the thoracic inlet and inferiorly by the lower border of the azygos vein. Typical endoscopic measurements from the incisors are from 20 to <25 cm.
The middle thoracic esophagus is bordered superiorly by the lower border of the azygos vein and inferiorly by the inferior pulmonary veins. Typical endoscopic measurements from the incisors are from 25 to <30 cm.
The lower thoracic esophagus is bordered superiorly by the inferior pulmonary veins and inferiorly by the stomach. Because it is the end of the esophagus, it includes the EGJ. Typical endoscopic measurements from the incisors are from 30 to 40 cm.
Clinical and Surgical Points of Interest
Recognition of the different landmark points and the distinct parts of the esophagus is of great help in localizing different lesions and pathological processes when performing barium swallow contrast radiograms and/or endoscopy. Such reference points are very useful to surgeons to plan access routes to the esophagus, such as a right thoracic approach for supracarinal lesions, and a left thoracic approach for lower third esophageal lesions. The lateral deviation of the esophagus has relevance, for example, in the placement of a cervical esophagostomy, treatment of Zenker’s diverticula, or fashioning of an esophagogastrostomy, most being performed on the left side of the neck.
The arbitrary 10-cm segment encompassing the distal 5 cm of the esophagus and proximal 5 cm of the stomach, with the EGJ in the middle, is an area of contention. In the new AJCC edition, cancers whose epicenter is in the lower thoracic esophagus, EGJ, or within the proximal 5 cm of the stomach (cardia) that extend into the EGJ or esophagus are stage grouped similar to adenocarcinoma of the esophagus. All other cancers with an epicenter in the stomach greater than 5 cm distal to the EGJ, or those within 5 cm of the EGJ but not extending into the EGJ or esophagus, are stage grouped using the gastric cancer staging system.
The Esophageal Sphincters
Upper Esophageal Sphincter (Fig. 6)
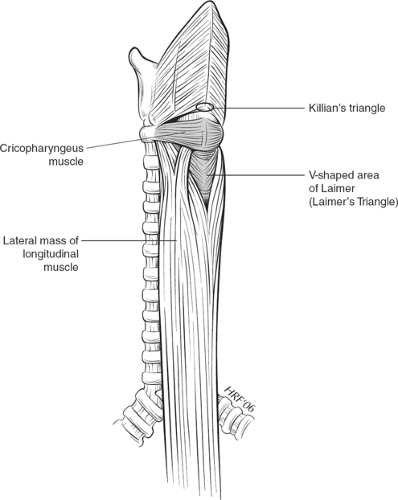
The cervical esophagus is approximately 6 cm long. The UES (upper esophageal sphincter) is a true anatomic sphincter that is composed of the dense cricopharyngeus muscle. When the cricopharyngeus muscle relaxes the upper esophageal sphincter opens aided by elevation and forward motion of the larynx. It is attached to the lamina of the cricoid cartilage (Fig. 6) and as it passes posteriorly it runs continuously without insertion into the midline raphe of the constrictors, the latter located cranial to it, thereby forming a sling. The cricopharyngeus muscle is considered by Last to be the lower transverse part of the inferior constrictor muscle of the hypopharynx. It blends into the circular fibers of the esophagus below. The longitudinal fibers ascending from the esophagus diverge at this point to form tendons on both sides, the central ones inserting into the posterior lamina of the cricoid cartilage. The posterior wall of the hypopharynx is relatively weak just above and below the cricopharyngeus. The Killian’s triangle comprises the area between the thyropharyngeus and cricopharyngeus muscles that comprise the inferior constrictor. Laimer’s triangle is an area that is formed between the cricopharyngeus muscle above and the divergent longitudinal esophageal muscle fibers below.
Clinical and Surgical Points of Interest
Sometimes and mostly in elderly patients a pharyngoesophageal pouch develops in Killian’s triangle, an example of a pulsion diverticulum. However, it is not a true diverticulum as not all layers are involved. The belief is that the pouch forms due to a lack of compliance of the upper esophageal sphincter and relative weakness of Killian’s triangle. Although the UES is anatomically short in height, that is, 0.5 cm, manometric measurements show the UES area to be much longer extending into the proximal part of the striated muscle of the cervical esophagus. A myotomy of the upper esophageal sphincter zone starting distal to the neck of the pouch therefore forms the most significant part of the repair for this condition, rather than excision or diverticulopexy. Importantly, the length of the myotomy
should be directed by the manometric not the apparent anatomic, that is, the length of the cricopharyngeal muscle, length. It is important during rigid or flexible esophagoscopy not to enter an esophageal pouch and risk perforation. Indeed, with osteophytes being prevalent at this part of the cervical spine, making esophagoscopy more difficult, this area of weakness is the commonest site of iatrogenic esophageal perforation.
should be directed by the manometric not the apparent anatomic, that is, the length of the cricopharyngeal muscle, length. It is important during rigid or flexible esophagoscopy not to enter an esophageal pouch and risk perforation. Indeed, with osteophytes being prevalent at this part of the cervical spine, making esophagoscopy more difficult, this area of weakness is the commonest site of iatrogenic esophageal perforation.
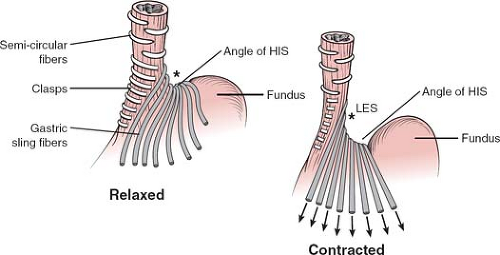 Fig. 7. Sling and clasp fibers of the LES, in contracted and relaxed states. Sharpening of the angle of His in contracted state. AH, angle of His. |
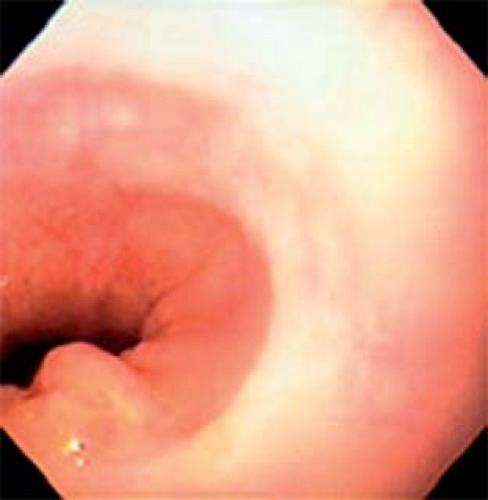 Fig. 8. Normal endoscopic view of the gastroesophageal junction showing the red velvet appearance of the gastric mucosa and folds and the pale color of the esophagus. |
Les and Gastroesophageal Junction (Fig. 7)
The LES, however, should be considered more a physiological sphincter than an anatomic one. It consists of an area of high intraluminal pressure—the high pressure zone—at the GEJ (gastroesophageal junction), and is influenced by a paracrine hormone effect and the intrinsic nervous system (see later).
The GEJ has anatomical features that provide a complex antireflux function. The latter includes the length of the intra-abdominal tubular esophagus, the acute angle that the esophagus subtends with the stomach, that is, the angle of His, the gaiter-like effect of the phrenoesophageal ligament, the pinching of the diaphragmatic crura, the sling and clasp fibers of the muscles in the wall (Fig. 7), and the rosette of gastric folds of mucosa around the EGJ.
Clinical and Surgical Points of Interest
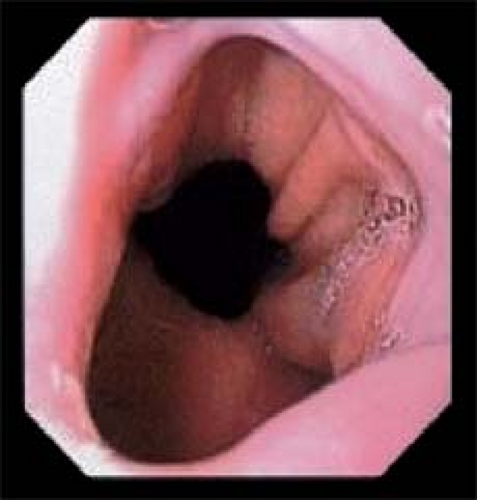
Endoscopically the LES can be recognized as a relative narrowing just proximal to the GEJ. The GEJ can be endoscopically recognized as the proximal margin of the gastric folds (Fig. 8) that in the majority of patients coincides with a pinching at the end of the tubular esophagus and the diaphragmatic indentation. Identifying these endoscopic landmarks allows a precise description of the GEJ, and the confirmation or ruling out of a sliding hiatus hernia. To do so one has to observe the relation between the proximal margin of the gastric folds and the indentation of the diaphragm (Fig. 9).
Research has evidenced a key element in preventing reflux is the restoration of 3 to 4 cm of distal esophagus under the diaphragm, according to Laplace law. This forms the anatomic basis of any type of antireflux surgery. In some patients, the pressure of the LES does not fall to normal resting values on relaxation. This, in conjunction with lack of coordination of the esophageal body, occurs in achalasia (see Nerve Supply Clinical and Surgical Points of Interest). Like the upper esophageal sphincter, the surgical treatment therefore consists of a myotomy of the last 4 to 6 cm of the esophagus of all layers down to the mucosa, extending 1 to 2 cm onto the stomach. There is discussion as to whether the myotomy should be extended only into the sling fibers or whether it should be performed more to the right and including the clasp fibers. This should be followed by a fundoplication to prevent reflux, classically an anterior (Dor) wrap 4 cm in length.
Tissue Architecture
Description
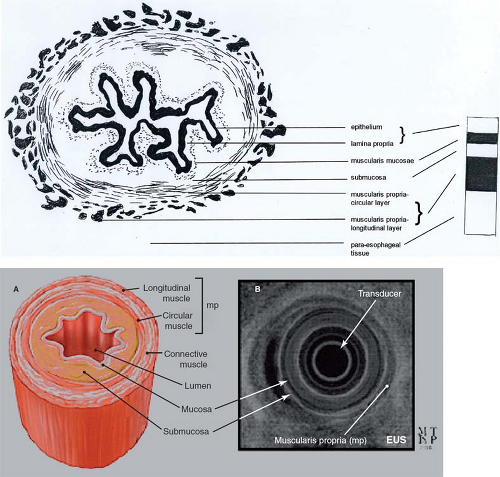
The esophagus seen in cross section consists of several layers (Fig. 10). The innermost layer is the mucosa, a nonkeratinizing
stratified squamous-lined epithelium, except at the gastroesophageal junction where the mucosa is composed of a columnar epithelium. Cell proliferation takes place in the basal layer lying on a basement membrane; differentiation and maturation occurs in the cells progressively, migrating more and more superficially. Next to this is the lamina propria, a layer containing blood vessels, lymphatic capillaries, and nerves. Both the epithelium and the lamina propria contain inflammatory and other cells: melanocytes, neuroendocrine cells, lymphocytes, and Langerhans cells. Adjacent to this layer is the muscularis mucosae that allow crinkling of the mucosa of the esophagus in an undistended state, then the submucosa, loose connective tissue with mucus-secreting glands, and clusters of specialized nerve cells—the Meissner’s plexus. Outside this is the muscularis propria or muscle coat, composed of two layers—a deep thicker circular and a superficial thinner longitudinal muscle layer with the myenteric or Auerbach’s plexus found between the two layers. The circular fibers run transversely at the superior and inferior ends of the esophagus but have a helicoidal course between the two. There are more longitudinal fibers laterally in the proximal esophagus, thus making this layer thicker here than the ventral or dorsal sides; however, it becomes more uniform more distally. The longitudinal layer courses in an elongated spiral, turning one quarter around the circumference of the esophagus. There is a loose adventitial covering to the muscular coat, but there is no true serosa and no mesentery unlike the rest of the distal gastrointestinal tract, except for the rectum. The esophagus can be thought of as consisting of two tubes, one riding almost independently over the other: the inner mucosa and submucosa separated by the muscularis mucosa; the outer muscularis propria.
stratified squamous-lined epithelium, except at the gastroesophageal junction where the mucosa is composed of a columnar epithelium. Cell proliferation takes place in the basal layer lying on a basement membrane; differentiation and maturation occurs in the cells progressively, migrating more and more superficially. Next to this is the lamina propria, a layer containing blood vessels, lymphatic capillaries, and nerves. Both the epithelium and the lamina propria contain inflammatory and other cells: melanocytes, neuroendocrine cells, lymphocytes, and Langerhans cells. Adjacent to this layer is the muscularis mucosae that allow crinkling of the mucosa of the esophagus in an undistended state, then the submucosa, loose connective tissue with mucus-secreting glands, and clusters of specialized nerve cells—the Meissner’s plexus. Outside this is the muscularis propria or muscle coat, composed of two layers—a deep thicker circular and a superficial thinner longitudinal muscle layer with the myenteric or Auerbach’s plexus found between the two layers. The circular fibers run transversely at the superior and inferior ends of the esophagus but have a helicoidal course between the two. There are more longitudinal fibers laterally in the proximal esophagus, thus making this layer thicker here than the ventral or dorsal sides; however, it becomes more uniform more distally. The longitudinal layer courses in an elongated spiral, turning one quarter around the circumference of the esophagus. There is a loose adventitial covering to the muscular coat, but there is no true serosa and no mesentery unlike the rest of the distal gastrointestinal tract, except for the rectum. The esophagus can be thought of as consisting of two tubes, one riding almost independently over the other: the inner mucosa and submucosa separated by the muscularis mucosa; the outer muscularis propria.
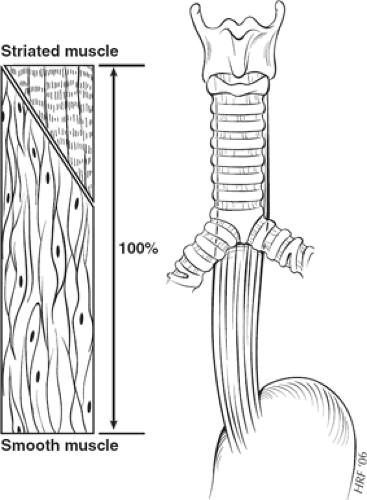
The esophagus can also be divided into thirds in terms of its muscle type. The proximal third consists purely of striated muscle fibers, the distal third entirely of smooth muscle fibers, and the middle third of a mixture of the two, with a greater proportion of smooth muscle fibers as the esophagus is followed caudally. The circular muscle fibers change from striated to smooth muscle type more proximally than those of the longitudinal muscle layer (Fig. 11).
Clinical and Surgical Points of Interest
Endoscopic ultrasound shows a characteristic alternation of hyperechoic and hypoechoic bands representing the different layers of the esophageal wall (Fig. 10), and aiding especially in the diagnosis of intramural and extramural masses as well as in distinguishing a T1a (intramucosal) from T1b (submucosal) tumors, standard esophagoscopy not being able to contribute this level of detail.
The helicoidal muscle course aids peristalsis and results in significant elastic recoil, thereby making it challenging to measure distances after resection and difficult, if not impossible, to resect an even short segment of esophagus and fashion an esophagoesophageal anastomosis, for example, in the case of trauma, short stricture, or small tumor. In addition, the mucosa can retract several millimeters inside the muscular coat, when the esophagus is transected, making it important to identify and catch both the mucosal and the muscle layer in each suture when performing an esophageal anastomosis.
It may be difficult to discriminate between true (organic) esophageal shortening caused by fibrosis due to chronic reflux esophagitis and that present in cases of—usually large—mixed hiatus hernia due to changes in elastic recoil caused by helicoidal retraction. The amount of shortening of the tubular esophagus in such hernias can be determined by measuring the distance between the distal border of the upper and proximal border of the LESs, endoscopically or manometrically determined. Contrast X-ray and endoscopic and manometric information are therefore required before embarking on laparoscopic repair of such a hernia to ensure success. Some authors consider a hiatal hernia of more than 5 cm as well as a large mixed hernia a contraindication for an abdominal—open or laparoscopic—approach.
Motility disorders of the esophagus do not involve the proximal esophagus as these are diseases affecting smooth muscle.
Barrett’s esophagus is a change of the tubular esophageal mucosa from a normal squamous epithelium. Endoscopically it is seen as a velvet red mucosa in the tubular esophagus. Moreover, there is variation in the height of the Z-line—the endoscopically determined transition between the whitish squamous epithelium and the red columnar epithelium, thus making the diagnosis of Barrett’s esophagus challenging. It is crucial therefore to describe where biopsies are taken from. For this, one must first accurately identify the GEJ (see earlier) and the proximal extent of the squamocolumnar junction, and tongues of Barrett’s metaplasia if present. According to the American College of Gastroenterology, the contemporary definition of Barrett’s esophagus requires two major components—a proximally displaced squamocolumnar junction as seen on endoscopy and the identification of acid-mucin–containing goblet cells, that is, specialized intestinal metaplasia (SIM) in a biopsy from the area of endoscopic abnormality. Based on the length of the columnar segment in the distal esophagus, Barrett’s esophagus has been arbitrarily divided into short segment (less than 3 cm) or long segment (equal to or more than 3 cm). Biopsies in these areas always require the presence of columnar epithelium (columnar epithelium lined esophagus (CELLO)) with presence of intestinal metaplasia. However, biopsies from a macroscopically normal-looking squamocolumnar junction can also contain intestinal metaplasia, considered to be the result of carditis. Studies have shown that progenitor cell populations (stem cells) in the basal layer of the squamous epithelium and in the duct epithelium of the esophageal glands may differentiate into a glandular phenotype, leading to the development of columnar epithelium in the distal esophagus. It is believed that this is the mechanism that results in the development of Barrett’s esophagus as the result of the healing process in patients suffering from reflux esophagitis, in particular in the presence of mixed acid and biliary reflux.
Arterial Supply