A. Epinephrine is a circulating hormone synthesized, stored, and released from the adrenal medulla. Its natural functions upon release into the circulation include regulation of myocardial contractility, heart rate, vascular and bronchial smooth muscle tone, glandular secretions, and metabolic processes such as glycogenolysis and lipolysis. It is a potent activator of α-adrenergic receptors and also activates β1 and β2 receptors. Oral administration is not effective as epinephrine is rapidly metabolized in the gastrointestinal mucosa and liver (administered subcutaneously, intravenously [IV], or intramuscularly). Epinephrine is poorly lipid soluble, preventing its ready entrance into the central nervous system and accounting for the lack of cerebral effects.
1. Clinical uses of epinephrine include treatment of life-threatening allergic reactions/anaphylaxis, treatment of severe asthma and bronchospasm, administration during cardiopulmonary resuscitation as a vital therapeutic drug, administration during periods of hemodynamic instability to promote myocardial contractility and increase vascular resistance, and continuous infusion for continuous support of myocardial contractility and vascular resistance. Epinephrine is added to local anesthetic solutions to decrease systemic absorption prolonging the duration of action of the anesthetic for regional and local anesthesia.
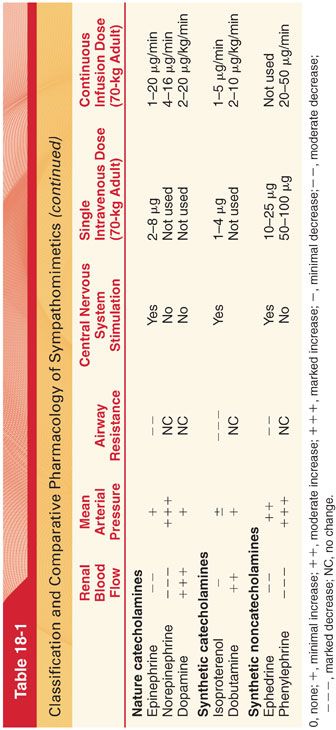
2. Cardiovascular Effects. The cardiovascular effects of epinephrine result from stimulation of α- and β-adrenergic receptors (see Table 18-1).
3. Airway Smooth Muscle. Smooth muscles of the bronchi are relaxed by epinephrine-induced activation of β2 receptors. The bronchodilating effects of epinephrine are not seen in the presence of β-adrenergic blockade.
4. Metabolic Effects. Epinephrine has the most significant effect on metabolism of all the catecholamines.
a. β1 Receptor stimulation due to epinephrine increases liver glycogenolysis and adipose tissue lipolysis, whereas α1 receptor stimulation inhibits release of insulin.
b. Release of endogenous epinephrine and the resulting glycogenolysis and inhibition of insulin secretion is the most likely explanation for perioperative hyperglycemia.
5. Electrolytes
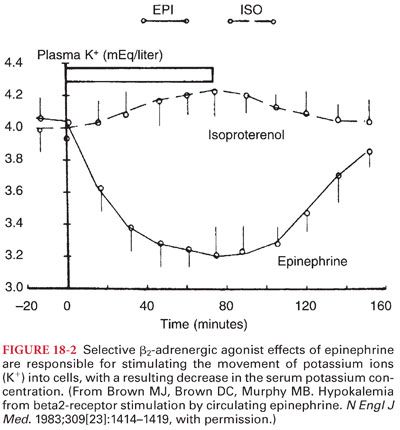
a. Selective β2-adrenergic agonist effects of epinephrine are speculated to reflect activation of the sodium-potassium pump in skeletal muscles, leading to a transfer of potassium ions into cells (Fig. 18-2).
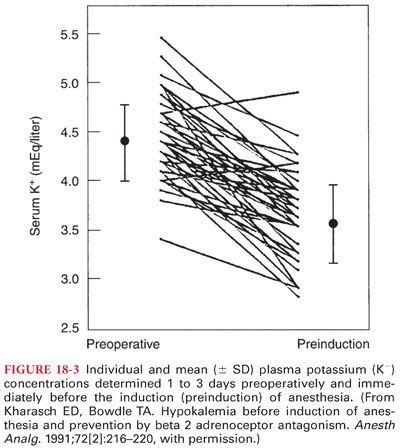
b. The observation that serum potassium measurements in blood samples obtained immediately before induction of anesthesia are lower than measurements 1 to 3 days preoperatively is presumed to reflect stress-induced release of epinephrine (Fig. 18-3). In making therapeutic decisions based on a preinduction serum potassium measurement, especially in patients without a reason to experience hypokalemia, one should consider the possible role of preoperative anxiety and the release of epinephrine.
6. Ocular Effects. Epinephrine causes contraction of the radial muscles of the iris, producing mydriasis.
7. Gastrointestinal and Genitourinary Effects
a. Epinephrine, norepinephrine, and isoproterenol produce relaxation of gastrointestinal smooth muscle.
b. Activation of β-adrenergic receptors relaxes the detrusor muscle of the bladder, whereas activation of α-adrenergic receptors contracts the trigone and sphincter muscles.
c. Hepatosplanchnic vasoconstriction occurs as well as impaired renal blood flow as cardiac output is diverted to the dilated skeletal muscle vasculature.
8. Coagulation is accelerated by epinephrine. A hypercoagulable state present during the intraoperative and postoperative period may reflect stress-associated release of epinephrine.
B. Norepinephrine is the endogenous neurotransmitter synthesized and stored in postganglionic sympathetic nerve endings and released with sympathetic nerve stimulation. Norepinephrine stimulates β1– and α1-adrenergic receptors (see Table 18-1). A continuous infusion of norepinephrine, 2 to 16 μg per minute, may be used to treat refractory hypotension.
1. Clinical Uses. The primary utility of norepinephrine is as a potent vasoconstrictor to increase total peripheral vascular resistance and mean arterial pressure. It is a first-line agent in the treatment of refractory hypotension during severe sepsis. Norepinephrine-induced vasoconstriction and redistribution of flow may increase splanchnic blood flow and urine output in severely hypotensive septic patients.
2. Side Effects. The use of norepinephrine as an inotropic agent is limited by its action as a potent vasoconstrictor. Excessive vasoconstriction and decreased perfusion of renal, splanchnic, and peripheral vascular beds may lead to end-organ hypoperfusion and ischemia.
C. Dopamine is an endogenous catecholamine that regulates cardiac, vascular, and endocrine function and is an important neurotransmitter in the central and peripheral nervous systems. Dopamine receptors may also be associated with the neural mechanism for “reward” that is associated with cocaine and alcohol dependence. Traditionally, the pharmacokinetics of dopamine has been attributed to dose-dependent effects on varying receptors (too simplistic as even in healthy individuals there are a wide range of clinical responses depending on individual variability in pharmacokinetics). Despite identical IV infusion rates, there may be a 10- to 75-fold variability in plasma dopamine concentrations produced even in healthy individuals with normal drug metabolism. The effects of dopamine cannot be predicted based on the dose, and the drug must be titrated to effect. Dopamine increases cardiac output by stimulation of β1 receptors, increasing stroke volume (less dysrhythmogenic than epinephrine). Rapid metabolism of dopamine with an elimination half-life of 1 to 2 minutes mandates its use as a continuous infusion (1 to 20 μg/kg/minute) to maintain therapeutic plasma concentrations.
1. Clinical Uses
a. Dopamine is used clinically to increase cardiac output in patients with decreased contractility, low systemic blood pressure, and low urine output as may be present after cardiopulmonary bypass or with chronic heart failure (unique among the catecholamines in being able to simultaneously increase myocardial contractility, renal blood flow, glomerular filtration rate, excretion of sodium, and urine output).
b. The divergent pharmacologic effects of dopamine and dobutamine make their use in combination potentially useful (infusions of dopamine and dobutamine produce a greater improvement in cardiac output, at lower doses, than can be achieved by either drug alone). The objective of combination therapy is to increase coronary perfusion and cardiac output while decreasing afterload, similar to an intraaortic balloon pump.
2. Renal-Dose Dopamine
a. The term renal-dose dopamine or low-dose dopamine refers to the continuous infusion of small doses (1 to 3 μg/kg/minute) of dopamine to patients to promote renal blood flow. In healthy individuals, low-dose dopamine increases renal blood flow and induces natriuresis and diuresis.
b. The term renal-dose or low-dose dopamine is misleading as dopamine has many effects at sites other than the kidneys, even at low doses.
c. In the absence of data confirming the efficacy of dopamine in preventing acute renal failure, renal-dose dopamine cannot be recommended.
3. Cardiovascular Effects. Dopamine is associated more than dobutamine or epinephrine with dose-related sinus tachycardia and the potential to cause ventricular arrhythmias and may predispose to myocardial ischemia by precipitating tachycardia, increasing contractility, increasing afterload, and precipitating coronary artery vasospasm.
4. Gastrointestinal Effects. There is no evidence that low-dose dopamine has beneficial effects on splanchnic function or reduces the progression to multiorgan failure in sepsis.
5. Endocrine and Immunologic Effects
a. Dopamine disrupts metabolic and immunologic functions through its effects on hormones and lymphocyte function.
b. In the acute phase of an illness, dopamine induces the pattern of hypopituitarism seen in prolonged critical illness and chronic stress. When dopamine is used in the chronic phase of illness, it further suppresses the circulating concentrations of pituitary hormones.
c. Dopamine’s overall effect is to suppress the secretion and function of anterior pituitary hormones, aggravating catabolism and cellular immune function and inducing central hypothyroidism.
6. Respiratory Effects. The infusion of low-dose dopamine interferes with the ventilatory response to arterial hypoxemia and hypercapnia, reflecting the role of dopamine as an inhibitory neurotransmitter at the carotid bodies (result is depression of ventilation in patients who are being treated with dopamine to increase myocardial contractility).
II. Synthetic Catecholamines (see Table 18-1) (see Fig. 18-1)
A. Isoproterenol is the most potent activator of all the sympathomimetics with β1 and β2 receptor activity (two to three times more potent than epinephrine and at least 100 times more active than norepinephrine, devoid of α agonist effects). The cardiovascular effects of isoproterenol reflect activation of β1 receptors in the heart and β2 receptors in skeletal muscle. Although cardiac output may increase thereby increasing systolic blood pressure, the mean arterial pressure may decrease due to decreases in systemic vascular resistance and associated decreases in diastolic blood pressure. Compensatory baroreceptor-mediated reflex slowing of the heart rate does not occur during infusion of isoproterenol because mean arterial pressure is not increased. Metabolism of isoproterenol in the liver by catechol-O-methyltransferase is rapid, necessitating a continuous infusion to maintain therapeutic plasma concentrations.
1. Clinical Uses. A continuous infusion of isoproterenol, 1 to 5 μg per minute, is effective in increasing the heart rate in adults in the presence of heart block. Isoproterenol is used to provide sustained increases in heart rate before insertion of a temporary or permanent cardiac pacemaker.
2. Adverse Effects. Vasodilation and decreased blood pressure may limit the use of isoproterenol. The combination of decreased diastolic blood pressure and increased heart rate and dysrhythmias may lead to myocardial ischemia.
B. Dobutamine is a synthetic catecholamine derived from isoproterenol consisting of a 50:50 racemic mixture of two stereoisomers. Dobutamine has potent β1-adrenergic effects with weaker β2-adrenergic activity. Its effect on α receptors increases at higher doses. Because dobutamine does not possess clinically important vasoconstrictor activity, it may be ineffective in patients who require increased systemic vascular resistance rather than augmentation of cardiac output to increase systemic blood pressure. Dobutamine affects heart rate through its action on β1-adrenergic receptors. Dobutamine stimulates sinoatrial node automaticity as well as atrioventricular nodal and ventricular conduction. At low doses, increases in heart rate may be minimal. However, high doses of dobutamine (>10 μg/kg/minute IV) may predispose the patient to tachycardia and cardiac dysrhythmias. Unlike dopamine, dobutamine does not act indirectly by stimulating the release of endogenous norepinephrine. Dobutamine does not activate dopaminergic receptors to increase renal blood flow. Renal blood flow, however, may improve as a result of drug-induced increases in cardiac output. Rapid metabolism of dobutamine (half-life of 2 minutes) necessitates its administration as a continuous infusion of 2 to 10 μg/kg/minute to maintain therapeutic plasma concentrations.
1. Clinical Uses
a. Dobutamine produces potent β-adrenergic agonist effects at doses less than 5 μg/kg/minute IV increasing myocardial contractility (β1 and α1 receptors) and causing a modest degree of peripheral vasodilation (β2 receptors).
b. Dobutamine is used to improve cardiac output in patients with congestive heart failure and is also useful for weaning from cardiopulmonary bypass. Vasodilators may be combined with dobutamine or dopamine to decrease afterload, optimizing cardiac output in the presence of increased systemic vascular resistance.
2. Adverse Effects. The use of dobutamine may be limited by the occurrence of tachyarrhythmias (occur more frequently at higher dosages or in patients with underlying arrhythmias or heart failure).
III. Synthetic Noncatecholamines (Fig. 18-4)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


