C. Classification (Tables 19-1 and 19-2)
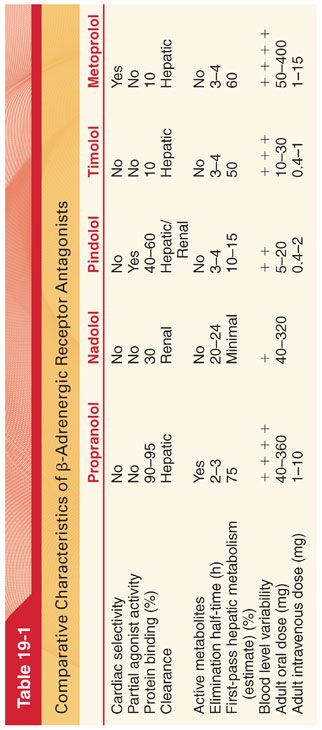
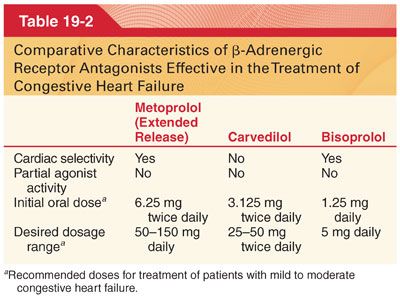
D. Pharmacokinetics (see Table 19-1)
1. The principal difference in pharmacokinetics between all the β-adrenergic receptor antagonists is the elimination half-time ranging from brief for esmolol (about 10 minutes) to hours for the other drugs.
2. β-Adrenergic receptor antagonists are eliminated by several different pathways and this must be considered in the presence of renal and/or hepatic dysfunction.
3. The therapeutic plasma concentration varies greatly among these drugs and between patients (interpatient variability). Explanations for interpatient variability include differences in basal sympathetic nervous system tone, flat dose-response curves for the drug so changes in plasma concentrations evoke minimal changes in pharmacologic effects, impact of active metabolites, and genetic differences in β-adrenergic receptors that influence how an individual patient responds to a given drug and plasma concentration.
E. Propranolol is a nonselective β-adrenergic receptor antagonist that lacks intrinsic sympathomimetic activity and thus is a pure antagonist (antagonism of β1 and β2 receptors is about equal) (see Table 19-1). As the first β-adrenergic antagonist introduced clinically, propranolol is the standard drug to which all β-adrenergic antagonists are compared. Typically, propranolol is administered in stepwise increments until physiologic plasma concentrations have been attained, as indicated by a resting heart rate of 55 to 60 beats per minute.
1. Cardiac Effects
a. Propranolol decreases heart rate and myocardial contractility by β1 receptor blockade, resulting in decreased cardiac output.
b. Heart rate slowing induced by propranolol lasts longer than the negative inotropic effects, suggesting a possible subdivision of β1 receptors.
c. Concomitant blockade of β2 receptors by propranolol increases peripheral vascular resistance, including coronary vascular resistance but drug-induced decreases in myocardial oxygen requirements predominate.
2. Pharmacokinetics. Propranolol is rapidly and almost completely absorbed from the gastrointestinal tract, but systemic availability of the drug is limited by extensive hepatic first-pass metabolism (may account for 90% to 95% of the absorbed dose). There is considerable individual variation in the magnitude of hepatic first-pass metabolism, accounting for up to 20-fold differences in plasma concentrations of propranolol in patients after oral administration of comparable doses. Hepatic first-pass metabolism is the reason the oral dose of propranolol (40 to 800 mg per day) must be substantially greater than the IV dose (0.05 mg/kg given in increments of 0.5 to 1.0 mg every 5 minutes).
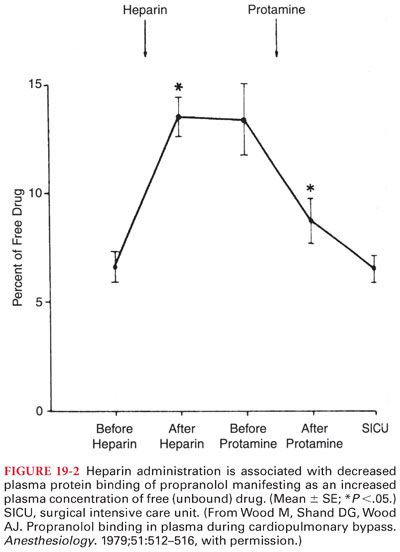
a. Protein Binding. Propranolol is extensively bound (90% to 95%) to plasma proteins. Heparin-induced increases in plasma concentrations of free fatty acids due to increased lipoprotein lipase activity result in decreased plasma protein binding of propranolol (Fig. 19-2).
b. Metabolism. Clearance of propranolol from the plasma is by hepatic metabolism. An active metabolite, 4-hydroxypropranolol, is equivalent in activity to the parent compound. Elimination of propranolol is greatly decreased when hepatic blood flow decreases. Renal failure does not alter the elimination half-time of propranolol, but accumulation of metabolites may occur.
c. Clearance of Local Anesthetics. Propranolol decreases clearance of amide local anesthetics by decreasing hepatic blood flow and inhibiting metabolism in the liver.
d. Clearance of Opioids. Pulmonary first-pass uptake of fentanyl is substantially decreased in patients being treated chronically with propranolol.
F. Nadolol and pindolol are nonselective β-adrenergic receptor antagonists; nadolol is unique in that its long duration of action permits once daily administration.
1. Pharmacokinetics. Nadolol is slowly and incompletely absorbed (an estimated 30%) from the gastrointestinal tract. Metabolism does not occur, with about 75% of the drug being excreted unchanged in urine and the remainder in bile.
G. Timolol is a nonselective γ-adrenergic receptor antagonist that is as effective as propranolol for various therapeutic indications. In addition, timolol is effective in the treatment of glaucoma because of its ability to decrease intraocular pressure, presumably by decreasing the production of aqueous humor. Timolol is administered as eye drops in the treatment of glaucoma, but systemic absorption may be sufficient to cause resting bradycardia and increased airway resistance.
1. Pharmacokinetics. Timolol is rapidly and almost completely absorbed after oral administration but extensive first-pass hepatic metabolism limits the amount of drug reaching the systemic circulation to about 50% of that absorbed from the gastrointestinal tract.
H. Metoprolol is a selective β1-adrenergic receptor antagonist that prevents inotropic and chronotropic responses to β-adrenergic stimulation, whereas bronchodilator, vasodilator, and metabolic effects of β2 receptors remain intact (metoprolol is less likely to cause adverse effects in patients with chronic obstructive airway disease or peripheral vascular disease). Selectivity is dose related, and large doses of metoprolol are likely to become nonselective, exerting antagonist effects at β2 receptors as well as β1 receptors (airway resistance may increase in asthmatic patients).
1. Pharmacokinetics. Metoprolol is readily absorbed from the gastrointestinal tract, but this is offset by substantial hepatic first-pass metabolism such that only about 40% of the drug reaches the systemic circulation.
I. Atenolol is the most selective β1-adrenergic antagonist that may have specific value in patients in whom the continued presence of β2 receptor activity is desirable. In patients at risk for coronary artery disease, treatment with IV atenolol before and immediately after surgery, followed by oral therapy during the remainder of the hospitalization, decreases mortality and the incidence of cardiovascular complications for as long as 2 years. Perioperative administration of atenolol to patients at high risk for coronary artery disease significantly decreases the incidence of postoperative myocardial ischemia.
1. Pharmacokinetics. Atenolol undergoes little or no hepatic metabolism and is eliminated principally by renal excretion.
J. Betaxolol is a cardioselective β1-adrenergic antagonist with no intrinsic sympathomimetic activity and weak membrane-stabilizing activity.
K. Bisoprolol is a β1 selective antagonist drug without significant intrinsic agonist activity. The most prominent pharmacologic effect of bisoprolol is a negative chronotropic effect. Bisoprolol is useful in the treatment of essential hypertension and has been shown to improve survival in patients with mild to moderate congestive heart failure (see Table 19-2).
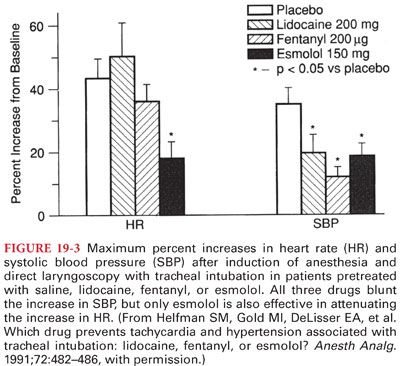
L. Esmolol is a rapid-onset and short-acting selective β1-adrenergic receptor antagonist that is administered only IV. After a typical initial dose of 0.5 mg/kg IV over about 60 seconds, the full therapeutic effect is evident within 5 minutes, and its action ceases within 10 to 30 minutes after administration is discontinued (useful for preventing or treating adverse systemic blood pressure and heart rate increases that occur intraoperatively in response to noxious stimulation, as during tracheal intubation). Esmolol, 150 mg IV, administered about 2 minutes before direct laryngoscopy and tracheal intubation provides reliable protection against increases in both heart rate and systolic blood pressure, which predictably accompanies tracheal intubation (Fig. 19-3).
1. Pharmacokinetics
a. Esmolol is available for IV administration only (only other β-adrenergic antagonists that may be administered IV are propranolol and metoprolol).
b. Plasma esterases responsible for the hydrolysis of esmolol are distinct from plasma cholinesterase, and the duration of action of succinylcholine is not predictably prolonged in patients treated with esmolol.
c. Evidence of the short duration of action of esmolol is return of the heart rate to predrug levels within 15 minutes after discontinuing the drug.
M. Side Effects. The side effects of β-adrenergic antagonists are similar for all available drugs, although the magnitude may differ depending on their selectivity and the presence or absence of intrinsic sympathomimetic activity. The principal contraindication to administration of β-adrenergic antagonists is preexisting atrioventricular heart block or cardiac failure not caused by tachycardia.
1. Cardiovascular System. β-Adrenergic antagonists produce negative inotropic and chronotropic effects. Patients with peripheral vascular disease do not tolerate well the peripheral vasoconstriction associated with β2 receptor blockade produced by nonselective β-adrenergic antagonists. The principal antidysrhythmic effect of β-adrenergic blockade is to prevent the dysrhythmogenic effect of endogenous or exogenous catecholamines or sympathomimetics.
a. Treatment of Excess Myocardial Depression. The usual clinical manifestations of excessive myocardial depression produced by β-adrenergic blockade include bradycardia, low cardiac output, hypotension, and cardiogenic shock. Excessive bradycardia and/or decreases in cardiac output due to drug-induced β blockade should be treated initially with atropine in incremental doses of 7 μg/kg IV. Atropine is likely to be effective by blocking vagal effects on the heart and thus unmasking any residual sympathetic nervous system innervation. If atropine is ineffective, continuous infusion of isoproterenol in doses sufficient to overcome competitive β blockade is appropriate. Glucagon administered to adults, 1 to 10 mg IV followed by 5 mg per hour IV, effectively reverses myocardial depression produced by β-adrenergic antagonists at normal doses because these drugs do not exert their effects by means of β-adrenergic receptors. In the presence of bradycardia that is unresponsive to pharmacologic therapy, it may be necessary to place a transvenous artificial cardiac pacemaker.
2. Airway Resistance. Nonselective β-adrenergic antagonists such as propranolol consistently increase airway resistance as a manifestation of bronchoconstriction due to blockade of β2 receptors (exaggerated in patients with preexisting obstructive airway disease).
3. Metabolism. Nonselective β-adrenergic antagonists such as propranolol interfere with glycogenolysis that ordinarily occurs in response to release of epinephrine during hypoglycemia. Tachycardia, which is an important warning sign of hypoglycemia in insulin-treated diabetics, is blunted by β-adrenergic antagonists (nonselective β-adrenergic antagonists are not recommended for administration to patients with diabetes mellitus who may be at risk for developing hypoglycemia).
4. Distribution of extracellular potassium across cell membranes is influenced by sympathetic nervous system activity as well as insulin (stimulation of β2-adrenergic receptors seems to facilitate movement of potassium intracellularly).
5. Interaction with anesthetics. Additive myocardial depression with β-adrenergic antagonists and anesthetics is not excessive, and treatment with β-adrenergic antagonists may therefore be safely maintained throughout the perioperative period (an exception may be patients treated with timolol in whom profound bradycardia has been observed in the presence of inhaled anesthetics).
6. Nervous System. β-Adrenergic antagonists may cross the blood–brain barrier to produce side effects (fatigue and lethargy). Atenolol and nadolol are less lipid soluble than other β-adrenergic antagonists and thus may be associated with a lower incidence of central nervous system effects.
7. Fetus. β-Adrenergic antagonists can cross the placenta and cause bradycardia, hypotension, and hypoglycemia in newborn infants of mothers who are receiving the drug.
N. Withdrawal Hypersensitivity. Acute discontinuation of β-adrenergic antagonist therapy can result in excess sympathetic nervous system activity that manifests in 24 to 48 hours. Presumably, this enhanced activity reflects an increase in the number of β-adrenergic receptors (upregulation) during chronic therapy with β-adrenergic antagonists.
O. Clinical Uses (Table 19-3). It is accepted that patients being treated with β-adrenergic receptor antagonists should have their medication continued uninterrupted through the perioperative period. It is also recommended that patients at high risk for myocardial ischemia and presenting for major surgery should be treated with β-adrenergic receptor antagonists beginning preoperatively and continuing into the postoperative period.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


