Fig. 12.1
Schematic illustration of (a) the applicator, (b) four quadrant electrode deployment sites (© 2012 Mederi Therapeutics Inc.)
The results of various series of the Secca® procedure reported in the literature are summarized in Table 12.1. The first reported experience with the Secca® procedure was by Takahashi et al. in 2002 [21]. In a pilot study of 10 patients with 12 months of follow-up, these researchers demonstrated a significant reduction in Wexner faecal incontinence scores, from 13.5 to 5.0. At 24 months of follow-up, the average Wexner score in this same cohort was 7.8 [23]. This was still a significant decrease from baseline but not as large as that seen at 1-year of follow-up. A multicenter, prospective, manufacturer-sponsored study in the United States conducted by Efron and colleagues included 50 patients who were followed for 6 months. The results of this study demonstrated a more modest decrease in Wexner scores from 14.5 to 11.1 [22]. However, the authors noted an improvement in all four components of the FIQL score as compared with baseline. Ruiz et al. noted a similar modest decrease in Wexner scores from 15.6 to 12.9 at 12 months in patients treated with the Secca® procedure [26]. Lefebure et al. reported a minimal change in Wexner scores in a series of patients followed for 12 months [25].
Table 12.1
Summary of trials on radiofrequency energy therapy (Secca® procedure)
Author (year) | n | Follow-up (months) | Wexner score | Comments | |
|---|---|---|---|---|---|
Before | After | ||||
Takahashi (2002) [20] | 10 | 12 | 13.5 | 5 | Four bleeding complications, 3 resolved, 1 required suture control |
Efron (2003) [21] | 50 | 6 | 14.5 | 11.1 | 18 % had prior overlapping sphincteroplasty; 4 % had prior artificial bowel sphincter |
Takahashi (2003) [22] | 10 | 24 | 13.7 | 7.8 | No significant difference between 1- and 2-year follow-up |
Takahashi (2008) [23] | 19 | 60 | 14.4 | 8 | Sustained benefit |
Lefebure (2008) [24] | 15 | 12 | 14.1 | 12.3 | No long-term complications. No change in FIQL scores except in the depression subscale |
Ruiz (2010) [25] | 16 | 12 | 15.6 | 12 | No long-term complications |
Because the device was unavailable for several years, recent data are limited. However, the Secca® procedure remains a viable option. It is a minimally invasive procedure that can be performed under local anaesthesia, and it has few associated complications. In patients with incontinence who do not have a known sphincter defect, the number of surgical options is limited. Further studies may help elucidate which of these patients may benefit most from the Secca® procedure.
Injectables
The symmetry and anatomy of the anal canal have been recognized as important components in the maintenance of continence. The use of injectable bulking agents for the treatment of faecal incontinence stems from similar technology in the field of urinary incontinence. Table 12.2 lists several of the early and more recent trials on injectable agents. Use of these agents is attractive due to their minimally invasive nature. First described for faecal incontinence by Shafik and colleagues in 1993, submucosal injection of polytetrafluoroethylene (Teflon or Polytef™; DuPont, Wilmington, NE) and autologous fat yielded successful short-term outcomes. Other injected agents include carbon-coated beads, silicone, collagen, nonanimal stabilized hyaluronic acid stabilized in dextranomer microspheres, polyacrylamide, and porcine dermal collagen.
Table 12.2
Summary of trials on injectable materials
Author (year) | n | Material used | Follow-up (months) | Wexner score | P value | |
|---|---|---|---|---|---|---|
Before | After | |||||
Shafik (1993) [26] | 11 | PTFE | 24 | 63 % improved | – | |
Shafik (1995) [27] | 14 | Autologous fat | 24 | 85 % improved | – | |
Malouf (2001) [28] | 10 | Bioplastique® | 6 | 30 % improved | _ | |
Davis (2003) [29] | 18 | Durasphere® | 28.5 | 11.8 | 8 | 0.002 |
Tjandra (2004) [30] | 82 | Silicone (US guided) | 12 | 14.5 | 3 | <0.001 |
Chan (2006) [31] | 7 | PTQ® | 14 | 9–14 | 1–5 | 0.016 |
Stojkovic (2006) [32] | 73 | Contigen® | 12 | 10 | 6 | <0.001 |
de la Portilla (2008) [33] | 20 | PTQ® | 24 | 13.5 | 9.4 | 0.127 |
Maeda (2008) [34] | 10 | Bulkamid® | 19 | 15 | 12 | <0.05 |
Maeda (2008) [34] | 10 | Permacol® | 19 | 16 | 15 | <0.05 |
Soerensen (2009) [35] | 33 | Silicone | 12 | 13 | 11 | – |
Tjandra (2009) [36] | 20 | PTQ® | 12 | 12 | 4 | <0.001 |
Graf (2011) [37] | 206 | NASHA/Dx | 12 | 14.3 | 10.9 | <0.001 |
The Cochrane Collaboration reviewed the available literature on injectable agents in 2010. Based on four randomized trials involving 176 patients, the authors observed a high risk for bias, which precluded definitive conclusions [39]. They did note, however, that most trials showed an improvement in patients’ symptoms in short-term follow-up. A small randomized trial comparing silicone biomaterial and carbon-coated beads showed a greater proportion of patients in the silicone group having a 50 % reduction in incontinent episodes at 12 months of follow-up [37]. The injection method employed in each of the reported studies is highly variable. In a study including 82 patients who underwent injection of silicone, Tjandra et al. reported significantly improved functional and quality of life outcomes with ultrasound-guided injections compared with non-guided injections [31].
Noting the relative lack of high-quality, randomized data, the NASHA Dx Study Group conducted a manufacturer-sponsored, international, multicenter, randomized, double-blinded controlled trial at 8 US centres and 5 European centres [38]. Patients with a Wexner score of greater than 10 and who had failed conservative management were randomized in a 2:1 fashion to transanal submucosal injection with nonanimal stabilized hyaluronic acid stabilized in dextranomer microspheres (Solesta®, Salix Pharmaceuticals, Raleigh, NC) or sham therapy, which consisted of mimicking the procedure without injection of any substance. The primary end point was a response to treatment as defined by a reduced number of incontinent episodes by 50 % or more compared with baseline. Fifty-two percent of patients in the treatment group achieved this end point at 6 months compared with 31 % of patients in the sham arm (odds ratio 2.36, 95 % CI 1.24−4.47, p = 0.009). There was no difference in the median decrease in number of incontinent episodes or change in Wexner scores from baseline between the treatment and sham groups. There was a significant difference between the treatment and sham groups in the mean increase in number of incontinence-free days at 6 months (3.1 vs 1.7, p = 0.016) [38].
The role of injectable materials for faecal incontinence has not yet been fully defined. However, their demonstrated efficacy, minimally invasive nature, and low complication rates are certainly advantageous attributes that may support the use of this therapy, either as a primary treatment of faecal incontinence or as an adjunct. The long-term results, optimal dose, and site of injection are all issues that remain to be clarified.
Replacement Muscle Transposition: Non-stimulated Gluteoplasty
Gluteus maximus muscle transposition was described in the early twentieth century as a treatment for faecal incontinence [40]. The gluteus maximus muscle is suitable for transposition due to its large bulk, proximity to the anal canal, and single proximal neurovascular pedicle. In addition, buttock contraction is a standard response to impending incontinence [41].
Technique
In the prone jackknife position, the lower portion of the gluteus maximus muscles and the fascia are individually mobilized from their origins on the ileum and sacrum. The neurovascular bundle is identified near the ischial tuberosity and preserved. The muscle strips are tunnelled underneath the skin and anchored to the contralateral gluteus maximus muscle on each side so that the anus is fully encircled [41].
Outcomes
Devesa et al. reported the largest series of bilateral gluteoplasties for faecal incontinence, in which 9 of 17 patients achieved normal control and the most common reported morbidity was infection of the perianal wound [42]. However, with the introduction of gracilis transpositions, enthusiasm for the gluteoplasty diminished [41].
Muscle Transposition: Non-stimulated Graciloplasty
In 1952, Pickrell described a novel surgical approach to treating children with faecal incontinence caused by neurologic and congenital disorders [43]. The advantages of transposition of the gracilis muscle include its superficial location, ease of mobilization, and lack of requirement for strength or range of motion [41].
Technique
The technique entailed removing the gracilis muscle from the thigh, wrapping it around the anus, and attaching the free end to the contralateral ischial tuberosity. Patients underwent training to gain voluntary control of muscle contraction and relaxation.
Outcomes
Corman reported long-term outcomes of non-stimulated graciloplasty; 11 of 14 patients in this study had fair to excellent results [44]. Christiansen et al. reported a series of 16 patients who underwent unstimulated gracilis transpositions, with over 80 % improvement [45]. In an attempt to improve outcomes, Kumar et al. performed bilateral gracilis transpositions in 10 patients. They observed a 90 % improvement in continence maintained for 2 years [46].
Muscle Transposition: Stimulated Graciloplasty
The non-stimulated graciloplasty technique suffered from faced with certain long-term limitations. Namely, the chronic contraction of the transplanted gracilis muscle caused fatigue and thereby compromised the patient’s sustained control of continence. The gracilis muscle is naturally composed of fast-twitch type II fibres that are easily fatigable. An approach to resolving this limitation became available in the early 1980s, when researchers demonstrated that the application of low-frequency electrical stimulation could, over time, transform type II fibres into slow-twitch, fatigue-resistant type I fibres [47]. In 1988, Baeten and coworkers used this technology to advance the procedure of stimulated graciloplasty. Their approach, which is also called dynamic graciloplasty, involved stimulating the gracilis muscle with a pulse generator. Tested on patients with fatigue-related suboptimal control following transposition, this stimulation was demonstrated to engender a neosphincter characterized by involuntary resting tone [41, 48].
Technique
Depending on whether a diverting stoma is created, the procedure of stimulated graciloplasty is carried out in two or three phases. In the first phase, the gracilis is removed from the thigh and wrapped around the anus to form a skeletal muscle ring; the severed distal portion is anchored to the contralateral ischial tuberosity (Fig. 12.2). The stimulator (Medtronic Inc, Minneapolis, MN) is implanted in the abdominal wall (Fig. 12.2c), with leads placed on the main trunk or in the intramuscular portion of the gracilis muscle, close to the nerve. The second phase of the procedure involves conditioning the muscle through the application of low-frequency neuromuscular stimulation, delivered at increasing levels over a period of 8 weeks. This process gradually changes the contractile properties of the gracilis muscle, transforming its easily fatigable fast-twitch fibres into fatigue-resistant slow-twitch fibres. In this phase, the patient also learns to use an external magnet in order to turn the stimulator on and off, thereby causing muscle contraction and relaxation, which facilitates the control of continence [41, 49]. If a diverting stoma must be created, additional operative interventions are required for its creation and subsequent closure.
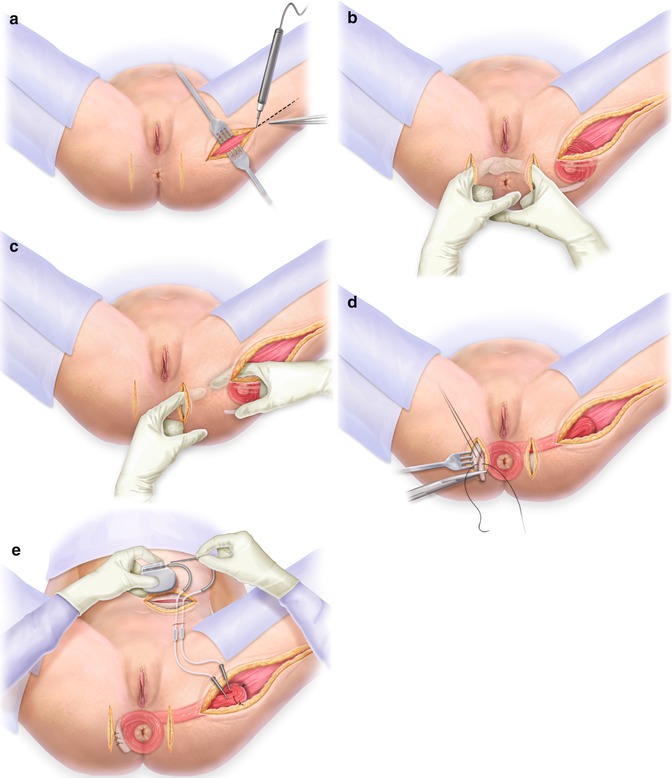

Fig. 12.2
(a) Incisions for harvesting the gracilis muscle. (b) Incision at the anus. (c) Incisions for tunneling the gracilis muscle from the leg to the anus. (d) Wrapping the gracilis muscle around the anus. (e) The stimulator that is implanted in the abdominal wall. With permission from Wolters Kluwer copyright 2012
Outcomes
Stimulated graciloplasty was a widely applied transposition procedure for treating faecal incontinence. Success rates generally range between 57 and 93 % [41, 49]. As reported in a prospective series including 17 patients, our initial experience with the procedure at Cleveland Clinic Florida indicated its feasibility despite a steep learning curve [50]. In an initial report of a multicenter trial, the Dynamic Graciloplasty Therapy Study Group (DGTSG) found that 60 % of patients who underwent the procedure had significant improvements in continence and quality of life [51]. Wexner et al. reported the long-term results of this multicenter trial, including outcomes of 129 patients who underwent stimulated graciloplasty from 1993 to 1999 [52]. Overall success, defined as a 50 % or greater reduction in the number of faecal incontinence episodes, was achieved in 62 and 56 % of patients at 1 and 2 years, respectively. These rates demonstrate the durability of the earlier, short-term DGTSG results. The authors of a systematic review reported that the efficacy of stimulated graciloplasty, as measured by patient reports of satisfactory continence, ranged from 42 to 85 % [53].
Many series have demonstrated that stimulated graciloplasty is effective in treating faecal incontinence; however, relatively high rates of complications and surgical revision have also been observed (Table 12.3). As reported by the DGTSG in the original trial, rates of complications and reoperations were 74 and 40 %, respectively [41, 57]. We observed a variety of complications associated with this procedure and its associated technology, including lead fibrosis, seroma of the thigh incision, excoriation of the skin above the stimulator, rotation of the stimulator, premature battery discharge, fracture of the lead, perineal skin irritation, perineal sepsis, rupture of the tendon, tendon erosion, muscle fatigue during programming sessions, electrode displacement from the nerve, and fibrosis around the nerve [50]. In addition, some patients who underwent the procedure had faecal impaction, anal fissure, and parastomal hernia. Other studies have reported instances of hardware failures, infections, muscle detachment, device malfunction and migration, postoperative evacuatory dysfunction, and severe unresolved pain that resulted in hospitalization and reoperation in numerous cases [53]. Some of these complications led to stoma creation or death.
Table 12.3
Outcomes of stimulated graciloplasty
Author (year) | n | Follow-up (months) | Morbidity (%) | Revision surgery (%) | Success (%) |
|---|---|---|---|---|---|
Christiansen (1998) [53] | 13 | 17 | – | – | 84 |
Sielezneff (1999) [54] | 16 | 20 | 50 | 43.7 | 81 |
Mavrantonis (1999) [55] | 21 IM | 21 | – | – | 93 |
6 DS | 12.5 | 10 | |||
Mander (1999) [56] | 64 | 10 | – | – | 56 |
Madoff (1999) [57] | 128 | 26 | 41 | – | 66 |
Konsten (2001) [58] | 200 IM | – | – | 2.7 | 74 |
81 DS | 26 | 57 | |||
Bresler (2002) [59] | 24 | – | 42 | 46 | 79 |
Wexner (2002) [51] | 129 | 24 | – | – | 62 |
Rongen (2003) [60] | 200 | 72 | – | 69 | 72 |
Penninckx (2004) [61] | 60 | 53 | 77 | 77 | 61 |
In 93 cases of dynamic graciloplasty, Matzel et al. reported 211 complications [63]. Although 42 % of patients in this study had severe complications, 92 % achieved recovery following treatment. With the exception of major infections, most of the complications did not adversely affect outcomes. In a systematic review of adverse events associated with the procedure, the most common complications observed were infection (28 %), device malfunction (15 %), and leg pain (13 %) [49, 53]. The mean morbidity rate was 1.12 per patient (range 0.14–2.08).
Risks of morbidity associated with stimulated graciloplasty have been reduced through selected modifications of the procedure. For example, rates of infectious complications have been lowered as a result of improved infection control measures [41, 61]. Complications caused by nerve fibrosis, lead displacement, and high impedance have been virtually eliminated by placing the leads adjacent to the intramuscular portion of the nerve rather than directly on the exposed portion of the nerve trunk [59, 61]. This method of stimulation is the only factor that has been identified as a significant predictor of successful outcomes of stimulated graciloplasty [59]. However, it is evident that surgical experience strongly impacts outcome [41, 58].
Despite evidence for the positive influence of stimulated graciloplasty on patient function and quality of life, Medtronic Inc. abandoned pursuit of FDA approval for the neurostimulator in the United States in 1999. This unfortunate decision was attributed partly to the relatively high rates of associated morbidity. Currently, surgeons in many other countries perform the operation to treat faecal incontinence. In addition, the technique of stimulated graciloplasty has been adapted for performing total anorectal reconstruction for anal atresia and following abdominoperineal resection. In the United States, sphincter muscle loss is currently treated with unstimulated graciloplasty. In the era of FDA approval for sacral nerve stimulation (SNS), graciloplasty still has a role in the treatment of faecal incontinence secondary to large sphincter defects or in patients with rectoanal atresia who are not likely to benefit from SNS and will continue to depend on sphincter replacement. Perhaps successful outcomes for this subgroup of patients might be best achieved with an artificial bowel sphincter preceded by a nonstimulated graciloplasty.
Artificial Bowel Sphincter
This approach to treating faecal incontinence involves implanting a prosthesis that simulates normal anal sphincter function. The Acticon® Neosphincter (American Medical Systems, Minnetonka, MN), which was approved by the FDA in 2001, is an implantable, fluid-filled artificial bowel sphincter (ABS) made of solid silicone rubber. The prosthesis comprises three parts that are connected with kink-resistant tubing: a perianal occlusive cuff, implanted around the anal canal; a pressure-regulating balloon, implanted in the abdomen; and a control pump with a septum, implanted in the labium majus or scrotum (Fig. 12.3) [31, 64, 65]. In response to the patient’s control of the pump mechanism, the Acticon® Neosphincter facilitates the opening and closing of the anal canal, simulating normal anal sphincter function. Continence is maintained as adjustments of the fluid-filled cuff compress the anal canal to a pressure approximating physiological resting values.
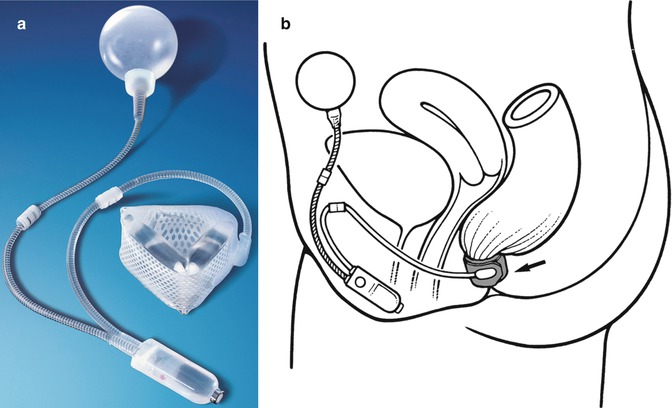

Fig. 12.3
(a) Artificial bowel sphincter system, (b) schematic of ABS placement for faecal incontinence in a female patient (Courtesy of American Medical Systems, Inc.)
In order to evacuate, the patient squeezes and releases the pump mechanism between 5 and 15 times. Each pump moves fluid from the cuff to the pressure-regulating balloon, thereby emptying and collapsing the cuff, which releases the compressive force around the anal canal. Due to residual pressure within the balloon, fluid passively flows back into the cuff, usually refilling it within 2–3 min. Continence is re-established as the refilled cuff compresses the anal canal again. Pressure in the occlusive cuff is maintained by the pressure-regulating balloon. The ABS device is available in different cuff lengths (8–14 cm), widths (2.0–2.9 cm), and balloon pressure ranges (80–120 cm H2O).
Technique
Because infection is a major complication of the ABS procedure, meticulous aseptic technique is imperative. Preoperatively, patients undergo a full bowel preparation and receive appropriate intravenous antibiotic prophylaxis. A modified lithotomy position is preferred in order to allow a combined perineal and abdominal approach. An anterolateral circumanal incision in the rectovaginal or rectourethral septum is created, and dissection proceeds in a cephalad direction. The ischiorectal fossae are entered on both sides, and a circumferential tunnel around the rectum is created, ideally proximal to the anococcygeal ligament, to minimize the chance of erosion through the perianal skin. This incision must be made very carefully to avoid injuring either the rectum or the vagina, as injury would preclude implantation of the artificial sphincter. A cuff sizer is passed around the anal canal to allow correct selection of the cuff width and length. The cuff itself is passed around the anus and fastened. A low transverse abdominal incision is made and a pocket in the space of Retzius is created. The cuff tubing is then tunneled up from the perineal incision towards this pocket. The balloon is inserted in the pocket. The control pump is implanted in the subcutaneous tissues of the scrotum in men and the labium majus in women on the ipsilateral side of the patient’s dominant hand. The balloon is filled with fluid and connected to the cuff tubing for 30 seconds to allow for pressurization of the cuff. The balloon is then drained and refilled with the appropriate amount of fluid. The final step is implantation and pressurization of the pump, tubing, and cuff via a colour-coded tubing system. The device is kept in the deactivated state until the surgical wounds have healed, generally over a period of 6 weeks after which it is activated in the outpatient setting.
Outcomes
Since Christiansen et al. first reported on ABS as a treatment for faecal incontinence in 1987, numerous case series have been published on the efficacy and safety of the procedure (Table 12.4) [31, 64, 82]. Wong et al. reported a large multicenter, prospective trial including 112 patients [76]. The findings demonstrated that the ABS is effective, achieving a continence rate of 85 % and a significant improvement in quality of life in patients with functioning devices. However, on an intention-to-treat basis, success was achieved in only 53 % of patients.
Table 12.4
Outcomes of artificial bowel sphincters
Author (year) | n | Follow-up (months) | Infection (%) | Device explant/reimplant | Functional (%) |
|---|---|---|---|---|---|
Wong (1996) [65] | 12 | 58 | 25 | 7/4 | 75 |
Lehur (1998) [66] | 13 | 30 | 8 | 4/2 | 85 |
Vaizey (1998) [67] | 6 | 10 | 33 | 1/0 | 83 |
Christiansen (1999) [68] | 17 | 60 | 18 | 7/0 | 53 |
Lehur (2000) [69] | 24 | 20 | 4 | 8/4 | 83 |
O’Brien (2000) [70] | 13 | – | 23 | 3/0 | 77 |
Altomare (2001) [71] | 28 | 19 | 18 | 5/0 | 75 |
Lehur (2002) [72] | 16 | 25 | 0 | 6/1 | 75 |
Devesa (2002) [73] | 53 | 26.5 | 21 | 12/2 | 49 |
Ortiz (2002) [74] | 22 | 28 | 9 | 9/2 | 68 |
Wong (2002) [75] | 112 | 12 | 38 | 41/7 | 67 |
Michot (2003) [76] | 25 | 34.1 | 12 | 5/0 | 76 |
Parker (2003) [77] | 37 | 12 | 19 | 27/7 | 49 |
Casal (2004) [78] | 10 | 29 | 10 | 3/2 | 90 |
Ruiz-Carmona (2008) [79] | 17 | 68 | 29 | 11/3 | 53 |
Wexner (2009) [80] | 47 | 39 | 41 | 18/4 | 65 |
Similar to stimulated graciloplasty, limitations to the ABS are attributable to its high rate of complications; most of these are related to infections of the foreign material and subsequent need for surgical revision and explantation. A systematic review of ABS case series reported a statistically and clinically significant improvement in Wexner and AMS scores after ABS implantation [83]. Six studies reported quality of life outcomes, which were also significantly improved compared with preoperative assessment. However, preoperative and postoperative functional and quality of life outcomes were not assessed by an intention-to-treat analysis in any of these studies; studies only compared outcomes in patients with a functional device. Thus, any negative impact of implantation followed by explantation of a failed ABS device was not assessed, possibly biasing the results. Ruiz-Carmona et al. reported long-term outcomes of ABS in 17 patients with a median follow-up of 5 years [80]. Only 9 of 17 (53 %) cases had an activated functional ABS by the end of the study period. However, those nine patients had significantly improved continence, with significant improvement in Wexner scores from 17.5 preoperatively to 9, 5.5 and 10 at 6, 12 months and at the end of follow-up, respectively. In addition, there was a significant improvement in quality of life.
Unfortunately, rates of postoperative complications of ABS have remained very high, ranging from 19 to 100 % [31, 64]. A systematic review including 14 studies reported explantation rates between 17 and 41 % and surgical revision rates between 13 and 50 % [83]. Wong et al. reported 117 postoperative complications in 114 cases [76]. The most common complications include infections, erosions or ulcerations of the rectum or surrounding skin, device malfunction such as cuff rupture, balloon and pump leaks, and device migration [76, 80, 81, 83, 84]. Fecal impaction, dehiscence of the perineal wound, pain, discomfort, and patient dissatisfaction are less common but also significant problems [81, 83, 84]. Among these complications, infection is the most common, resulting in explantation in 4–38 % of cases [76, 80, 81, 83, 84]. Ruiz-Carmona reported 5-year follow-up data in 17 patients with an ABS [80]. All patients suffered from at least one complication, and 65 % required at least one reoperation. After the first implant, 11 devices had to be removed (65 %) and 7 patients eventually underwent a second implantation.
ABS infection can be divided into two groups: early-stage infection, defined as infection prior to ABS activation, and late-stage infection, defined as infection after ABS activation [81, 83]. In a study from our institution, 21 of 51 patients (41.2 %) developed infection at a mean follow-up period of 39 months [81]. Eighteen (35.3 %) cases developed infection before ABS activation (early-stage infection), a result similar to other reports; all 18 cases required ABS explantation. A bowel movement prior to the third postoperative day and a history of perianal infection prior to ABS implantation were risk factors for early-stage ABS infection. In a study by Winslett et al., secondary procedures were necessary in up to 32 % of the patients treated for perianal abscess, either because of inadequate examination under anaesthesia for drainage or undefined occult fistula-in-ano [85].
Late-stage complications after ABS device activation can also result in ABS explantation. The incidence of these complications may increase with device use over time [78, 86]. In a series from our institution, the most common late-stage complication was device malfunction, followed by erosion of device, persistent perianal pain, migration of device, constipation, and hematoma over the labium majus [81]. This was similar to the other reports [74, 76, 78, 83, 84, 86]. Thirteen of 33 patients (32.0 %) required ABS explantation. In the late stage, device malfunction was the most common reason for explantation (46.1 %). All of the ABS devices that required explantation functioned satisfactorily after activation, but the function deteriorated with time. Four of 9 malfunctioned ABS devices were due to leakage of the system, which implied that the present design may be inadequate for longer-term function. Erosion of the rectum or skin was the second most common reason for explantation (38.5 %). Five patients had erosions through the skin and one had erosion through the rectum with associated rectovaginal fistula. Five patients had associated infection that resulted in explantation [81]. Tejirian et al. reported intra-abdominal erosion of artificial bowel sphincter reservoir [87]. Devesa et al. attempted to identify risk factors of erosion, but they found no association with pre-existing fibrosis, perianal wound closure method, tension of the wound, and soiling or straining during evacuation [74]. Similarly, we did not find erosion to be associated with any important patient-related factors [81]. Erosions were found only after ABS activation. The rate of explantation increased with the time after ABS implantation; the longer the ABS was in use, more complications occurred and the risk of ABS explantation increased. The 1-year and 2-year cumulative risks of ABS explantation were 9.7 and 13 %, respectively. After 2 years, the risk of ABS explantation sharply increased, and in the third and fourth year, risk increased to 43 and 48 %, respectively [81]. A similar explantation rate of 44 % at 48 months was also reported by Ortiz et al. [75].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


