Surgical Treatment of Laryngeal Cancer: A Legacy of Minimally Invasive Innovation and the Preservation of Airway, Swallowing and Vocal Function
Steven M. Zeitels
John C. Wain
Introduction
Successful surgical management of laryngeal cancer requires that the clinician integrate a complex algorithm of interdependent host and tumor issues. The patient and surgeon must mutually consider the optimal treatment modality. This is based on the efficacy of cure along with the potential detrimental effects of those interventions on airway patency, voice, and swallowing. The functional outcomes must be assimilated into patients’ age, vocal needs, and pulmonary reserve, as well as the skill sets of the surgeon, availability of technology, and prior oncological history of the patient. These concepts were initiated in the 19th century and progressively established through the 20th century due to the increasing frequency of laryngeal cancer subsequent to the introduction of mass-produced cigarettes.
The surgical treatment of laryngeal cancer during the last two centuries comprises a rich history chronicling the development of minimally invasive endoscopic laryngeal surgery, upper airway management, swallowing rehabilitation, and voice preservation. Independent and interdependent advances have led to current philosophies in which curative treatment is routine with serviceable airway and swallowing function while optimal vocal outcome remains a pursuit.
History and Development of Glottic Cancer Treatment: the 19Th Century
Horace Green: the First Direct Laryngoscopic Resection of A Laryngeal Neoplasm
The first endolaryngeal resection of a laryngeal neoplasm can be traced to the monumental achievements of Horace Greene (Fig. 1A, B) in the 1840s.
Having been first to routinely treat the trachea transorally, Green’s skill and confidence mounted so that his next achievement became one of the most important in human airway management. He was faced with a child who had developed obstructive apnea with accompanying stridor. Using a bent-tongue spatula, along with sunlight for illumination, he performed the first direct laryngoscopy.
Having successfully viewed the laryngeal introitus directly, he observed a ball-valving fibroepithelial polyp that was obstructing the glottal aperture. He proceeded to use a curved forceps to remove the mass. This became the first visually controlled endoscopic resection of a laryngeal lesion, which predated the routine use of mirror laryngoscopy and mirror-guided endolaryngeal surgery.
The Early Cures for Laryngeal Cancer
Remarkably, several years after Green’s triumph, Garcia presented mirror laryngoscopy, which catalyzed the formal development of laryngology by Czermak and Turck. Laryngology became highly developed as a result of office-based indirect endolaryngeal diagnoses and procedures.
During this period, Solis Cohen (Fig. 2) likely achieved the first cure of laryngeal cancer by performing a laryngofissure and cordectomy for early glottic cancer (1869). To the best that the author can determine he is the
first surgeon to specialize in laryngology having been trained by Samuel Gross and serving the Union as a general surgeon for 4 years of the Civil War. Although there was intermittent use of laryngofissure and cordectomy with varied success in the late 19th century, the procedure was established by Semon, Butlin, and Jackson. Billroth (Fig. 3) performed the first total laryngectomy for laryngeal cancer (1874). Although Billroth successfully achieved the resection, Solis Cohen and Gluck perfected the procedure by suturing the trachea to the skin and separating the airway from the pharyngo-esophagus.
first surgeon to specialize in laryngology having been trained by Samuel Gross and serving the Union as a general surgeon for 4 years of the Civil War. Although there was intermittent use of laryngofissure and cordectomy with varied success in the late 19th century, the procedure was established by Semon, Butlin, and Jackson. Billroth (Fig. 3) performed the first total laryngectomy for laryngeal cancer (1874). Although Billroth successfully achieved the resection, Solis Cohen and Gluck perfected the procedure by suturing the trachea to the skin and separating the airway from the pharyngo-esophagus.
In 1884, Koller and Jelinek introduced topical cocaine for mucosal anesthesia, which became a key chemical platform technology that greatly advanced office-based laryngeal surgery. Soon thereafter, Fraenkel (Fig. 4) performed the first successful transoral endoscopic resection of glottic cancer using mirror guidance. It is possible that this represents the first minimally invasive endoscopic cancer cure as well. There were further similar isolated reports over the next 30 years. This early endoscopic cancer treatment achievement did not significantly alter management strategies for glottic cancer, which were primarily comprised of transcervical open laryngectomy procedures.
 Fig. 2. Jacob Solis Cohen (1838–1927). (Photograph circa 1868, courtesy of Thomas Jefferson University, Archives & Special Collections, Scott Memorial Library, Philadelphia.) |
History and Development of Glottic Cancer Treatment: the 20Th Century
Formal Direct Laryngoscopic Treatment of Glottic Cancer
In 1895, Kirstein (Fig. 5) reintroduced direct laryngoscopy, described tracheoscopy, and established this technique as a routine surgical methodology. It can be argued that this advancement was the most influential in the history of laryngology given the ensuing development of general endotracheal anesthesia, cardiopulmonary resuscitation, and critical care management. Pursuant to our specialty, direct laryngoscopy became the foundation for a majority endoscopic cancer treatment of the upper aerodigestive tract. Kirstein also predicted that Oertel’s laryngeal stroboscope would be combined with direct laryngoscopy to enhance patient management of vocal-fold lesions. It was the promise of increased precision associated with direct laryngoscopy and commensurate advancements in anesthesia that subsequently promulgated the migration of direct endolaryngeal cancer surgery to the operating room, where most complex endolaryngeal surgery was done in the 20th century.
Killian (Fig. 6) acknowledged Kirstein’s achievements and based on this work introduced rigid bronchoscopy and suspension laryngoscopy. After modifying the Killian suspension laryngoscope, Lynch (Fig. 7) published a series of 39 patients whose early glottic cancers were excised en bloc by suspension laryngoscopy. This became the first substantial series of endoscopic glottic cancer resections. He carefully selected lesions that were exposed adequately, of small volume, limited to a single vocal cord, and did not extend to the anterior commissure or vocal process. Due to the technical difficulties of suspension laryngoscopy without general endotracheal anesthesia, this approach remained obscure.
Jako commenced experiments with laser technology to remove human tissue in the mid-1960s. By the early 1970s, Jako, Strong, and Vaughan were likely the first surgeons
to remove human tissue with a laser and shortly thereafter employed this groundbreaking technology to resect glottic cancer. Thirty years later, Zeitels et al. introduced angiolytic laser treatment of glottic cancer based on Folkman’s principles of neoplastic angiogenesis.
to remove human tissue with a laser and shortly thereafter employed this groundbreaking technology to resect glottic cancer. Thirty years later, Zeitels et al. introduced angiolytic laser treatment of glottic cancer based on Folkman’s principles of neoplastic angiogenesis.
Glottic Cancer
Disease Presentation and Philosophy of Management
In the United States, glottic cancer comprises <6,500 of the new <10,000 laryngeal cancer cases per annum. Hoarseness is the primary presenting symptom, however, with larger neoplasms, patients may also report airway restriction, discomfort, hemoptysis, dysphagia, odynophagia, and referred ear pain (otalgia). Glottic cancer is unique as compared with other sites of the upper aerodigestive tract due to the low incidence of regional metastasis and the ease in which the disease is cured locally if the correct surgical procedure is selected.
The success of local control of the primary tumor arises from the fact that the anatomy of the larynx is a relatively isolated “box.” It is bounded by a thick cartilaginous frame and dense connective tissue that are resistant to local and regional cancer spread while creating a series of self-contained compartments. This structural composition facilitates a variety of transoral (endoscopic) and transcervical (open neck) partial laryngectomy procedures that strive to preserve the three most critical functions of the larynx: (a) patent adequate airway caliber, (b) competent valve to preclude aspiration during deglutition, and (c) phonation and voice.
Glottic carcinoma is unique as the site of an organ cancer since very small lesions (2 mm) or even precancerous dysplasia can cause a discernable functional deficit, hoarseness. Furthermore, in the overwhelming majority of patients, voice loss is the sole or dominant symptom, which is easily recognizable to friends and family. Since local control is routine and aspiration can typically be avoided, the primary metrics for success in management are airway patency and voice restoration/preservation.
Due to the relative paucity of lymphatics in the true vocal folds, it is uncommon for patients to present with regional metastasis with T1 and T2 disease. Therefore, small- to mid-sized glottic cancers are ideal for single-modality endoscopic minimally invasive treatment. Remarkably, even those with T3 and T4 lesions often do not have associated adenopathy. However, the surgical treatment majority of T3 and T4 glottic cancers require an open (transcervical) resection with removal of some or all of the cartilage framework (total laryngectomy).
Laryngeal cancer is staged using the standard TNM classification system (Table 1) based on the subsite affected (supraglottis, glottis, or subglottis). As with other cancers, the TNM staging has prognostic value for survival. As treatment decisions are often made based on the staging of the cancer, it is valuable to become familiar with this system.
Surgery Versus Radiation
The two definitive treatment modalities for glottic carcinoma are radiation therapy (XRT) and surgery. Surgical therapy for glottic cancer can be divided into open or endoscopic techniques. Unlike radiotherapy, surgery treats only the areas of disease without ablating and distorting the remaining normal anatomy. Glottic cancer surgery can be done with millimeter margins thereby decreasing surgical morbidity by sparing normal tissue several millimeters away from the tumor margin. Therefore, surgical resection of selected glottic cancer can be performed with phonosurgical techniques, which allow for precise control of disease with preservation of the vocal ligament, thyroarytenoid muscle, and the superficial lamina propria (SLP). Accordingly, postoperative voice results are excellent, especially when phonosurgical reconstruction is done. Other advantages of surgery include lower cost, diminished time of intercurrent disease, and a very low complication rate. Unlike XRT, endoscopic treatment preserves all treatment modalities including further endoscopic management.
Radiotherapy treatment of early glottic cancer successfully controls local disease in a majority of cases; however, this advantage is mitigated by the fact that XRT is a single-use treatment and patients with laryngeal cancer frequently have metachronous neoplasms. However, despite a century of success with higher cure rates employing transoral and transcervical function-sparing surgical procedures, glottic carcinoma is typically treated with external beam radiotherapy (XRT) in a majority of western countries. T1 and T2 diseases are treated with radiotherapy alone while T3 and T4 lesions are typically treated with XRT and chemotherapy. The primary advantage of this approach is uniformity of management not necessitating highly individualized skills, a key requisite of optimal surgical interventions.
For early disease, the disadvantages of XRT include damage to noncancerous glottal tissue including (e.g., contralateral vocal fold), which is the primary voice source as well as ablation of the saccular glands, which are vital for lubrication of the glottis to facilitate vocal-fold vibration. For advanced disease, it is commonplace for radiation and chemotherapy to cause airway swelling and/or stenosis requiring a tracheotomy as well as severe dysphagia and/or pharyngo-esophageal stenosis, both of which are usually not present pretreatment and extremely difficult to resolve.
Radiation also commonly causes chronic mucositis often masking recurrence while resulting in discomfort, dryness, and taste changes. Less common, patients develop frank osteoradionecrosis of the laryngeal cartilages resulting in any of the aforementioned functional symptoms as well as wound infections. Finally, it is well recognized that a majority of the undesirable effects of radiation therapy are long lasting and often irreversible.
In the latter 20th century, radiotherapy became the mainstay of treatment for most stages of laryngeal cancer with chemotherapy used adjunctively. This was in part due to the fact that surgeons lack confidence that they could achieve optimal functional results. However, it is now been recognized that there is substantially diminished survival in a variety of stages if surgery is not employed as a primary initial treatment modality. Furthermore, complications and poor functional outcomes (voice, swallowing, and airway) from the nonsurgical regimens are being increasingly recognized. For both initial and salvage treatments, this has catalyzed new opportunities for surgeons
skilled in endoscopic approaches for small- to mid-sized tumors as well as open partial techniques for larger tumors. The endoscopic methods for supraglottic primaries will likely be further advanced by robotic innovation due to frequent restrictions in optimal laryngoscopic exposure.
skilled in endoscopic approaches for small- to mid-sized tumors as well as open partial techniques for larger tumors. The endoscopic methods for supraglottic primaries will likely be further advanced by robotic innovation due to frequent restrictions in optimal laryngoscopic exposure.
Table 1 TNM Staging of Laryngeal Cancer | |||||||
|---|---|---|---|---|---|---|---|
|
Classical Endoscopic Glottic Surgery for Early Cancer
The overarching key to successful endoscopic treatment of laryngeal cancer is obtaining the best possible laryngoscopic exposure, which comprised of optimal anatomic positioning along with the largest well-designed speculum and laryngoscope holder. Toward that end, we methodically described the key principles for success as well as designing a universal modular glottiscope and a new suspension gallows.
The goal of endoscopic treatment of early glottic cancer is eradication of the disease with maximal preservation of the normal layered microstructure. This approach results in the optimal postoperative voice without compromising oncologic cure. There are four basic procedures that are based on the depth of treatment (Fig. 8): (a) dissection just deep to the epithelial basement membrane and superficial to the superficial lamina propria for epithelial atypia and microinvasive cancer, (b) dissection within the superficial lamina propria microinvasive cancer that is not attached to the vocal ligament, (c) dissection between the deep lamina propria (vocal ligament) and the vocalis muscle for lesions that are attached to the ligament but not through it, and (d) dissection within the thyroarytenoid muscle for lesions penetrating the vocal ligament and invading the vocalis. This approach can be fine-tuned further by performing partial resections of any of the layered microstructure.
In the classical surgical paradigm, if dissection is performed in the SLP, cold instruments facilitate precise tangential dissection around the curving vocal fold. This allows for maximal preservation of the superficial lamina propria and for pliability of the regenerating epithelium. Dissection between the vocal ligament and the vocalis muscle can be performed equally well with cold instruments alone or with assistance by a laser. Dissection within the muscle is performed most precisely with a cutting laser, which allows for improved visualization because of hemostatic cutting properties.
Subepithelial saline-epinephrine infusion into Reinke’s space improves pre-excisional assessment of lesion depth. If the tumor has invaded the vocal ligament, the SLP at the
perimeter of the lesion will distend creating a contour depression in the region of the cancer. The subepithelial infusion assists with the surgeon’s technical execution of the surgery in a number of other ways: (a) The infusion facilitates mucosal incisions by improving visualization of the lateral border of the lesion and by distending the SLP so that the overlying epithelium is under tension. (b) The infusion also increases the depth of the superficial lamina propria, which facilitates less traumatic dissection in this layer and leads to regenerated epithelium that is more pliable. (c) The epinephrine and hydrostatic pressure of the infusion vasoconstricts the microvasculature in the SLP and this improves visualization and precise dissection. (d) If a laser is used, the saline acts as a heat sink, which decreases thermal trauma to the normal vocal-fold tissue.
perimeter of the lesion will distend creating a contour depression in the region of the cancer. The subepithelial infusion assists with the surgeon’s technical execution of the surgery in a number of other ways: (a) The infusion facilitates mucosal incisions by improving visualization of the lateral border of the lesion and by distending the SLP so that the overlying epithelium is under tension. (b) The infusion also increases the depth of the superficial lamina propria, which facilitates less traumatic dissection in this layer and leads to regenerated epithelium that is more pliable. (c) The epinephrine and hydrostatic pressure of the infusion vasoconstricts the microvasculature in the SLP and this improves visualization and precise dissection. (d) If a laser is used, the saline acts as a heat sink, which decreases thermal trauma to the normal vocal-fold tissue.
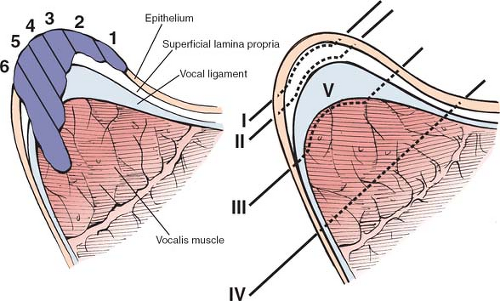 Fig. 8. Coronal section of the mid-vocal fold displaying. A: different potential depths of tumor invasion. B: Classification of different resection depths. |
When substantial soft tissue of the glottis was resected, it was commonplace to have some vocal dysfunction due to aerodynamic glottal insufficiency. During the 1990s, we developed a variety of procedures to reconstruct the glottal valve, which were adopted domestically and abroad. The lost paraglottic soft tissue was augmented with fat and/or Gore-tex and the anterior commissure tendon was reconstructed by means of an anterior laryngofissure and thyroid-lamina subluxation. However, restoring phonatory mucosal pliability remains a challenge.
After a decade of research, we have designed a promising vocal biogel and expect to commence human trials in 2012. This vocal biogel retains the possibility of restoring millions of voices including hundreds of thousands hoarse laryngeal cancer patients. It will likely even facilitate voice enhancement in patients who have undergone partial or total laryngectomy. In the latter, the mucosal vibration associated with esophageal speech and phonation with tracheo-esophageal valve speech will be made more effective.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree