Subtotal Gastrectomy for Gastric Cancer
John T. Mullen
Although the incidence of gastric cancer in the United States has dramatically decreased during the past several decades, gastric cancer remains an important cause of cancer-related death, with an estimated 21,000 cases and 10,600 deaths in 2009. Despite significant improvements in staging modalities, surgical therapy, and perioperative care, the prognosis of most patients with gastric cancer remains poor. This is largely a result of the aggressive biology of this cancer as well as the advanced stage of disease at which most patients present to the clinician. Risk factors include Helicobacter pylori infection, pernicious anemia, prior gastric resection, smoking, and high salt intake. Approximately 10% of gastric cancers are due to inherited cancer syndromes, including hereditary diffuse gastric cancer (HDGC), characterized by mutations in the E-cadherin (CDH1) gene, and hereditary nonpolyposis colorectal cancer (HNPCC) syndrome, characterized by mutations in DNA mismatch repair genes. This chapter focuses on the preoperative diagnostic and staging evaluation of the patient with gastric cancer as well as the technical maneuvers involved in the performance of a distal subtotal gastrectomy for this disease.
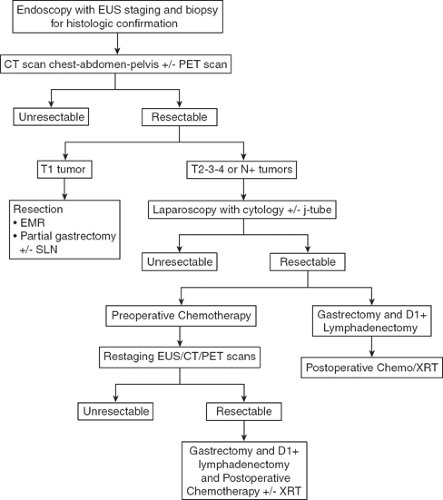
The evaluation of the patient with suspected gastric cancer includes a careful physical examination, laboratory studies, H. pylori status, endoscopic ultrasound (EUS), computed tomography (CT) and positron emission tomography (PET) scans, and laparoscopy. My current algorithm for the diagnosis and treatment of patients with gastric cancer is illustrated in Figure 1. The diagnosis of gastric cancer is usually readily established by endoscopy with biopsy. Endoscopy defines the location and extent of the tumor within the stomach and, when combined with EUS, allows for accurate estimation of the depth of tumor invasion (T stage). EUS is the most sensitive modality in establishing the T stage of a gastric cancer, and EUS enables an assessment of the regional lymph nodes and needle biopsy of suspicious nodes to confirm nodal involvement (N stage). A CT scan of the chest, abdomen, and pelvis should be performed to evaluate for distant metastatic spread, such as to the lungs, liver, peritoneum, or lymph nodes outside the field of resection. In addition, a CT scan is sensitive in detecting even small amounts of ascites that may represent peritoneal tumor spread and that can be sampled by image-guided means for cytologic examination. Though PET and PET–CT scans are not routinely recommended in the preoperative staging of gastric cancer, they may certainly provide additional useful information. PET has a low sensitivity in detecting the primary tumor, especially in early and diffuse-type gastric cancers, but PET has a higher specificity than CT (∼90% vs. ∼60%) in the detection of regional lymph node metastases, and PET has a reasonable sensitivity for the detection of liver, lung, and distant lymph node metastases. In addition, PET can be used to monitor tumor response to neoadjuvant chemotherapy, as it accurately detects responders to therapy at an early stage, thus enabling the clinician to maintain patients on as active a chemotherapy regimen as possible. Nonetheless, PET has shown variable success at best in predicting histopathological response and long-term survival.
Patients who should be considered for immediate resection include those with early, superficial (Tis, T1) gastric cancers and those who require immediate palliation of bleeding or high-grade tumor-associated luminal obstruction. However, the perioperative morbidity and mortality rates in this latter population of patients are significant and must be carefully weighed against the likely benefits of resection. Patients with more invasive lesions revealed
on EUS are considered for additional pretreatment staging by laparoscopy. Staging laparoscopy can be done immediately prior to the planned laparotomy or as a pretreatment procedure performed in patients considering preoperative therapy. Staging laparoscopy upstages up to 25% of patients through the identification of radiographically occult peritoneal and liver metastases and positive cytology. It is a particularly high-yield procedure for those patients with tumors involving the gastroesophageal junction (GEJ) or whole stomach. For patients considering preoperative chemotherapy or chemoradiation therapy, a feeding jejunostomy tube is often placed at the time of staging laparoscopy.
on EUS are considered for additional pretreatment staging by laparoscopy. Staging laparoscopy can be done immediately prior to the planned laparotomy or as a pretreatment procedure performed in patients considering preoperative therapy. Staging laparoscopy upstages up to 25% of patients through the identification of radiographically occult peritoneal and liver metastases and positive cytology. It is a particularly high-yield procedure for those patients with tumors involving the gastroesophageal junction (GEJ) or whole stomach. For patients considering preoperative chemotherapy or chemoradiation therapy, a feeding jejunostomy tube is often placed at the time of staging laparoscopy.
The most common treatment paradigm for gastric cancer in the United States has historically been upfront surgery, followed by adjuvant chemoradiation therapy (as described in the Intergroup 0116 trial) for those patients with high-risk (T3,4, and/or node positive) tumors. However, I favor perioperative chemotherapy for such patients, as described in the MAGIC trial conducted by the British Medical Research Council. In this trial, patients were randomized to receive either perioperative chemotherapy (three cycles of epirubicin, cisplatin, and 5-fluorouracil (ECF) preoperatively and postoperatively) and surgery or surgery alone. Five-year survival rates were superior in the perioperative chemotherapy group compared with the surgery-alone group (36% vs. 23%). Administration of at least a few cycles of systemic therapy in advance of surgery for patients with high-risk tumors (i.e., T3/4, node-positive, diffuse-type, GEJ location) confers several advantages: first, it permits early treatment of possible micrometastatic disease; second, one can monitor the in vivo tumor response to the therapy, such as with PET–CT scan imaging; and, third, one can select those patients with particularly bad tumor biology who develop early metastatic disease and who would thus derive no survival benefit from gastrectomy.
Surgical Considerations
A complete margin-negative (R0) resection remains the only potentially curative treatment for gastric adenocarcinoma. The choice of operation depends on the location of the tumor as well as the stage of disease. Superficially invasive gastric cancers of favorable histologic grade can be treated by endoscopic mucosal resection or wedge excision with or without concomitant sentinel lymph node biopsy with excellent results. Such procedures have been extensively described by our surgical colleagues from Japan, where gastric cancers are frequently diagnosed at an early stage, given the prevalence of screening endoscopic examinations in that country. Because the majority of patients in the United States present with symptomatic, more advanced tumors, the primary surgical question is which procedure offers the greatest chance for cure with acceptable morbidity and mortality. Many patients are not candidates for any surgical procedure either because they are medically unfit or because of the presence of metastatic disease seen on preoperative imaging studies or at the time of laparoscopy. For those patients who are candidates for gastric resection, the options include total, proximal subtotal, and distal subtotal gastrectomy. There is an increasing worldwide experience in performing these procedures laparoscopically, with improved short-term outcomes and nodal yields that are on par with the traditional open procedures. However, there is little evidence comparing the long-term outcomes of laparoscopic gastrectomy versus open gastrectomy, and the vast majority of resections for gastric cancer worldwide are still performed via the open approach. The indications for and technique of laparoscopic gastrectomy are discussed elsewhere in this volume. This chapter focuses on the technical issues of an open, distal, subtotal gastrectomy; yet before I address these issues, there are three major surgical considerations that merit discussion.
Extent of Luminal Resection
The extent of gastric resection is determined by the location and extent of the primary tumor. Prospective, randomized trials failed to demonstrate a survival advantage for total gastrectomy over distal, subtotal gastrectomy for patients with tumors of the distal stomach. Therefore, for those patients in whom a 5- to 6-cm margin from the tumor can be obtained, while still maintaining
a reasonably sized gastric remnant, a more conservative gastric resection should be performed, as this confers an equivalent survival rate with less morbidity and a better quality of life than does a total gastrectomy. Nonetheless, a total gastrectomy should be performed if necessary to achieve an R0 resection, as positive resection margins (R1 resections) lead to very poor survival. In the Dutch gastric cancer trial, 10% of patients had a positive resection margin and a correspondingly inferior 3-year survival (18% vs. 63%) compared with those who had a negative resection margin. However, microscopically involved margins appear to affect long-term survival only in those patients with five or fewer lymph node metastases.
a reasonably sized gastric remnant, a more conservative gastric resection should be performed, as this confers an equivalent survival rate with less morbidity and a better quality of life than does a total gastrectomy. Nonetheless, a total gastrectomy should be performed if necessary to achieve an R0 resection, as positive resection margins (R1 resections) lead to very poor survival. In the Dutch gastric cancer trial, 10% of patients had a positive resection margin and a correspondingly inferior 3-year survival (18% vs. 63%) compared with those who had a negative resection margin. However, microscopically involved margins appear to affect long-term survival only in those patients with five or fewer lymph node metastases.
Extent of Lymph Node Dissection
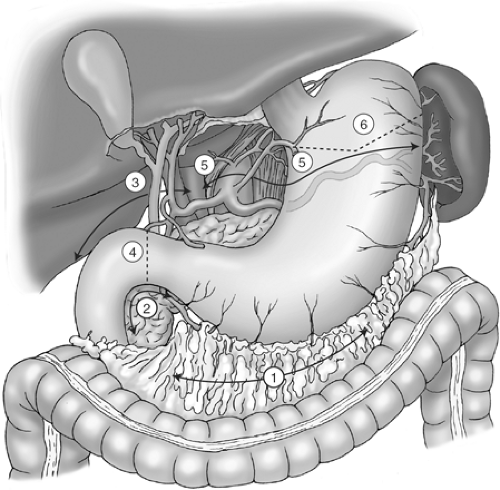
The extent of lymph node dissection is one of the most controversial issues in the management of gastric cancer. Retrospective studies from Japan suggest that performing an extended lymphadenectomy improves survival and demonstrate that it can be done safely, with a perioperative mortality rate of less than 1%. The Japanese Research Society for Gastric Cancer categorizes the draining lymph node basins of the stomach into 16 stations, including 6 perigastric stations and 10 regional stations along the major vessels and adjacent to the pancreas (Fig. 2A,B). The extent of lymph node dissection is indicated by the designation D: a D1 dissection includes only the perigastric nodes (stations 1 to 6); a D2 dissection includes the lymph nodes along the common hepatic, left gastric, celiac, and splenic arteries (stations 7 to 11); and a D3 dissection includes additional nodes within the porta hepatis and adjacent to the aorta (stations 12 to 16).
Two large, prospective randomized trials comparing the outcomes of D1 dissection with those of D2 dissection have been conducted in Western patients. Long-term follow-up of the larger of these two studies, namely, the Dutch Gastric Cancer Group Trial, demonstrated no long-term overall survival benefit with the D2 lymph node dissection (35% vs. 30%, P 0.53). In addition, there were significantly higher rates of postoperative morbidity (43% vs. 25%) and mortality (10% vs. 4%) in those patients undergoing the more extensive D2 lymphadenectomy, in large part resulting from the greater numbers of splenectomy and pancreatectomy performed in this group in order to achieve a complete node dissection. Similarly, the British Cooperative trial conducted by the Medical Research Council reported an increased morbidity, associated with high rates of concomitant splenectomy and pancreatectomy, without a benefit in overall or recurrence-free survival in those patients randomized to a D2 dissection.
Despite the findings of these two studies, investigators have argued that if the complication rate after an extended D2 lymph node dissection could be decreased, the survival benefit conferred by a D2 dissection, as reported by experienced centers in Korea and Japan, might be translated to Western patients with gastric cancer. In fact, analysis of the group that did not undergo a pancreatectomy or splenectomy in the Dutch trial showed a significant survival advantage for those patients who underwent a D2 dissection (11-year survival 47% vs. 33%, P 0.018). Thus, the Italian Gastric Cancer Study Group conducted a phase II trial of pancreas-preserving D2 lymphadenectomy at high-volume centers by surgeons who completed rigorous training in the technique through observation of master surgeons in Japan. They reported impressively low morbidity (20.9%) and mortality (3.1%) rates in patients undergoing the D2 dissection, in which a pancreatectomy or splenectomy was performed only for direct tumor invasion, as well as an overall 5-year survival rate of 55%. A phase III trial designed to demonstrate an improved survival in patients randomized to a D2 dissection versus a D1 dissection was launched by this group in 1998, and the results are still pending.
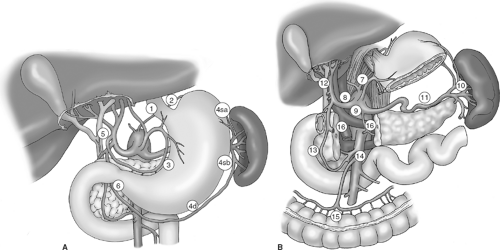 Fig. 2. Lymph node stations including (A) the perigastric, or D1, lymph nodes (stations 1 to 6), and (B) the regional, or D2 and D3, lymph nodes (stations 7 to 16). |
It remains unclear whether a D2 lymph node dissection is simply a thorough staging procedure or whether there may be a therapeutic benefit for a subset of patients with localized disease (e.g., patients with
pN2 disease). It should be noted that the accurate staging of patients with gastric cancer, according to the most recent seventh edition of the American Joint Committee on Cancer (AJCC) Staging Manual, demands the evaluation of at least 15 lymph nodes. The nodal staging is then based on the number of positive nodes, with N1 (1 to 2 positive nodes), N2 (3 to 6 positive nodes), N3a (7 to 15 positive nodes), and N3b (16+ positive nodes) categories. Studies have shown a correlation between improved patient survival and larger numbers of lymph nodes examined in the specimen. Of course, this may simply reflect more accurate staging as opposed to a therapeutic benefit from the procedure. However, there is indirect evidence that more extensive lymphadenectomies result in lower rates of locoregional recurrence, and this may translate into a survival benefit. Indeed, a randomized trial conducted at a single center in Taiwan identified an overall 5-year survival advantage for those patients undergoing a D3 dissection (59.5%) compared with those undergoing a D1 dissection (53.6%). However, a recently conducted trial by the Japan Clinical Oncology Group, in which patients undergoing an R0 resection are randomized at the time of surgery either to a D2 lymphadenectomy alone or with a para-aortic (D3) nodal dissection, demonstrated no difference in survival between the groups.
pN2 disease). It should be noted that the accurate staging of patients with gastric cancer, according to the most recent seventh edition of the American Joint Committee on Cancer (AJCC) Staging Manual, demands the evaluation of at least 15 lymph nodes. The nodal staging is then based on the number of positive nodes, with N1 (1 to 2 positive nodes), N2 (3 to 6 positive nodes), N3a (7 to 15 positive nodes), and N3b (16+ positive nodes) categories. Studies have shown a correlation between improved patient survival and larger numbers of lymph nodes examined in the specimen. Of course, this may simply reflect more accurate staging as opposed to a therapeutic benefit from the procedure. However, there is indirect evidence that more extensive lymphadenectomies result in lower rates of locoregional recurrence, and this may translate into a survival benefit. Indeed, a randomized trial conducted at a single center in Taiwan identified an overall 5-year survival advantage for those patients undergoing a D3 dissection (59.5%) compared with those undergoing a D1 dissection (53.6%). However, a recently conducted trial by the Japan Clinical Oncology Group, in which patients undergoing an R0 resection are randomized at the time of surgery either to a D2 lymphadenectomy alone or with a para-aortic (D3) nodal dissection, demonstrated no difference in survival between the groups.
So in light of these conflicting data, what is one to conclude about the appropriate extent of lymph node dissection for gastric cancer in Western patients? I recommend performing an “over-D1” (or “D1+”) lymphadenectomy, in which all D1 (perigastric) lymph nodes and as many D2 lymph nodes as are predicted to harbor metastatic disease, and that can be safely removed without the need for splenic or pancreatic resection, are included with the gastrectomy specimen. The Maruyama computer program may prove to be a helpful preoperative tool in this regard, as it accurately predicts the risk of lymph node metastasis in each nodal station based on the input of eight variables, including tumor histology, diameter, depth of invasion, and location. Failure to dissect those regional lymph nodes harboring metastatic disease, as predicted by the Maruyama program, will generate a high Maruyama Index (MI) of unresected nodal disease, which in retrospective analyses has been shown to result in higher rates of regional recurrence and inferior survival.
Extent of Adjacent Organ Resection
The value of extended organ resection for advanced gastric cancer is another area of great debate, particularly in light of the increased morbidity and mortality accompanying such resections as shown in the Dutch and British trials previously mentioned. However, in order to achieve an R0 resection in patients with T4 lesions, it is often necessary to resect additional organs en bloc with the stomach. With careful patient selection, limiting gastrectomy with additional organ resection to those with clinical T4 tumors without evidence of visceral, peritoneal, or extensive regional nodal disease, long-term survival with acceptable perioperative morbidity and mortality is possible. Additional organ resection is not an adverse predictor of survival in this group of patients; rather, the depth of tumor invasion and nodal stage are the primary determinants of survival.
Subtotal Gastrectomy and D2 Lymphadenectomy
Exploration
The patient is positioned supine on the operating room table and at the time of induction a single dose of a broad-spectrum antibiotic (e.g., a second-generation cephalosporin) is administered. The abdomen is entered via a generous vertical midline incision extending from the xiphoid process to just below the umbilicus. Although a bilateral subcostal incision affords excellent exposure of the upper abdomen as well, I prefer a midline incision, as this is associated with less postoperative pain and is more suitable should a combined thoracic approach (e.g., Ivor–Lewis procedure) be necessary. The falciform ligament is divided and retractors are placed on the abdominal wall with the aid of the Thompson or Bookwalter retractor system.
The abdomen is then carefully explored to evaluate both the extent of locoregional disease and the presence of distant metastases. Special attention should be focused on all peritoneal surfaces as well as the liver.
Sequence of Operative Procedures
The sequence of operative steps of a subtotal gastrectomy and D2 lymphadenectomy is illustrated in Figure 3 and includes the following:
Mobilization of the greater curvature with omentectomy and division of the left gastroepiploic vessels
Infrapyloric mobilization with ligation of the right gastroepiploic vessels
Suprapyloric mobilization with ligation of the right gastric vessels
Duodenal transection
D2 lymphadenectomy, with dissection of the porta hepatis, common hepatic artery, left gastric artery, celiac axis, and splenic artery, and ligation of left gastric vessels
Gastric transection
Reconstruction by loop or Roux-en-Y gastrojejunostomy
Greater Curvature Mobilization
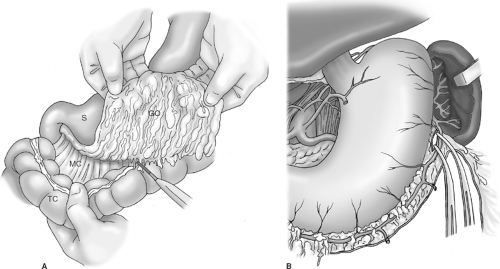
The greater omentum is dissected off the transverse colon with the aid of electrocautery (Fig. 4A). This step should be bloodless if dissection is correctly carried out in the avascular plane between the omentum and appendices epiploicae of the colon. I do not routinely resect the anterior leaf of peritoneum of the transverse mesocolon or the peritoneum overlying the anterior pancreas unless dictated by local tumor invasion. It is unrealistic to believe that routine resection of these layers comprising the omental bursa (bursectomy) confers a survival advantage by potentially removing microscopic peritoneal metastases. The omentum is resected with the specimen, as it contains numerous perigastric (D1, station 4) lymph nodes and is essentially devascularized during the course of the gastrectomy.
With the greater omentum reflected superiorly, dissection commences on the left side of the abdomen such that the omentum is dissected off of the splenic flexure and the inferior pole of the spleen, exposing the body and tail of the pancreas. The left gastroepiploic vessels are identified and divided near their origin from the splenic vessels (Fig. 4B). The spleen is carefully elevated into the operative field with moist packs placed posterior to it, and omental and colonic attachments to the spleen are lysed with the electrocautery (if needed) to prevent inadvertent capsular tears during the course of the dissection. Note that for a distal subtotal gastrectomy, preservation of the short gastric vessels is critical because this operation entails division of all four main arteries supplying the gastric remnant. In fact, one of the purported reasons for the unacceptably high morbidity and mortality rates reported in the Dutch Trial is that a number of patients who underwent distal gastrectomy also had ligation of the short gastric vessels as part of the mandated splenectomy to achieve a complete node dissection. Ligation of the short gastric vessels devascularizes the gastric remnant with resultant ischemia of the gastrojejunal anastomosis, and this perhaps explains the high anastomotic leak rate reported in this trial. A related point is that if during the course of a distal subtotal gastrectomy a splenic injury occurs, such that a splenectomy is necessary, a total gastrectomy should be performed in order to avoid the risk of necrosis of the gastric remnant.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree