Fig. 2.1
Classification of stem cells: Depending upon the differentiation potential into different lineages, stem cells are categorized as totipotent, pluripotent, multipotent, oligopotent, and unipotent
1.
Totipotent: A single cell capable of dividing and forming various differentiated cells including extraembryonic tissues is known as totipotent or omnipotent cell, e.g., a zygote.
2.
Pluripotent: The pluripotent stem cells have the ability to differentiate into all cell types of the three germ layers, i.e., ectoderm, mesoderm, and endoderm. Inability to form extraembryonic tissues such as placenta is the only limitation that makes them inferior to totipotent stem cells, e.g., embryonic stem (ES) cells.
3.
Multipotent: Stem cells that demonstrate a restricted pattern of differentiation toward few lineages are termed as multipotent cells such as hematopoietic stem cell (HSC), which can develop into various types of blood cells but not into brain or liver cells.
4.
Oligopotent: Oligopotent stem cells are able to differentiate into few cell types of specific lineages such as lymphoid stem cells that can be differentiated only into basophil, neutrophil, eosinophil, monocyte, and thrombocytes.
5.
Unipotent: Unipotent stem cells can only differentiate into one particular cell type such as hepatoblasts forming hepatocytes.
Besides normal developmental functions in multicellular organisms, these mother cells are believed to be the holy grail of medical therapy with high promise for regenerative medicine.
2.2 Engineering of Stem Cells
Stem cells with their unique differentiation potential may act as therapeutic tool to cure diseases which are beyond treatment with routine drug therapy including genetic disorders. Since stem cells are able to generate functionally active healthy cells/tissues, ailments such as neurodegenerative diseases, cardiovascular diseases, liver failure, diabetes, and renal failure where unhealthy cells that are at fault may achieve significant alleviation by stem cell therapy (Fig. 2.2). Current medical practices have not adapted these new therapy regimes routinely as most of them are under clinical trials and not approved yet. The main challenges involve modulation of stem cells toward lineage-specific differentiation in vitro and in vivo, monitor the differentiation, and finally assess the success rate in clinic. Preliminary results appear to be quite promising, which are discussed in the following sections.
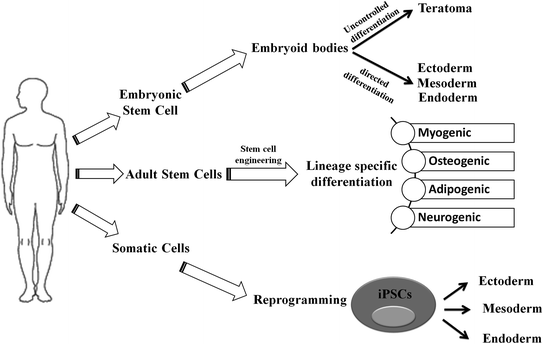

Fig. 2.2
Schematic representation of differentiation process of embryonic stem cell (ESC), adult stem cell (ASC), and induced pluripotent stem cells (iPSCs): While ESCs can generate three germ layers via directed differentiation, it may give rise to teratomas through uncontrolled differentiation in vivo. The ASCs have restricted potential to generate myogenic, osteogenic, adipogenic, and neurogenic lineages under specified conditions. In contrary to ESC and ASC that only differentiate toward specific lineage, iPSCs follow a dedifferentiation (from somatic cell) and then differentiation to germ-layer-specific cellular lineages
2.2.1 Human Embryonic Stem Cells
The unique potential of human ESCs (hESCs) to differentiate into three main germ layers and subsequent to any cell type of human body brought them into forefront of biomedical research with a caveat of forming teratoma in vivo during differentiation. However, recent progress in cell fate control, directed differentiation, and tissue engineering crossed the boundaries of laboratory and extended to the arena of regenerative medicine. hESC lines are conventionally derived from the inner cell mass (ICM) of preimplantation-stage blastocysts, morula-stage embryos, or late-stage blastocysts and express pluripotency markers (transcription factors—Oct4, Sox2, Nanog; surface antigens—SSEA-4, SSEA-3; proteoglycans—TRA-1-60, TRA-1-81) [1]. To maintain the undifferentiated state, these cells are co-cultured with a support or feeder layer derived from mouse embryonic fibroblast (MEF) that provides all the essential growth factors [1]. The reported success rate for hESC derivation is highly variable, possibly due to variation in embryo quality and culture conditions. Major progress had happened in derivation, propagation, cryopreservation, and efficient passaging of hESCs. Since clinical application of hESCs critically depends on well-characterized growth and differentiation of stem cells, much effort was put in developing conditioned media that will enable feeder-free growth of ESC cells and eliminate animal products [2, 3]. The original culture system for the maintenance of hESC using MEF feeder cell layer support possesses risk of zoonosis transmitted by animal pathogens, potential activation of animal retroviruses, and possibility of immune rejection due to the presence of nonhuman sialic acid. Several approaches such as use of extracellular matrix (ECM) derived from MEF than living feeder cells, hESC-derived fibroblasts, defined culture medium containing components solely derived from purified human material, MEF-conditioned Matrigel layer to establish and maintain clinical-grade hESCs cell lines are in progress [4]. However, these xeno-free and feeder-independent culture systems are costly and laborious and may lead to abnormal karyotype during long-term culture. Recently, Akopian et al. in conjunction with the Internal Stem Cell Initiative Consortium (ISCIC) compared several commercially available ESC culture media with Knockout Serum Replacer, FGF-2, and MEF cell layers for propagation of several hESC cell lines established in five different laboratories and showed that only mTeSR1 and STEMPRO were able to support most cell lines up to 10 passages [2].
The next major challenge for translational application of hESC is to direct their differentiation toward a specific cell lineage. The pioneer study by Itskovtz-eldor et al. [5] showed that hESC cells were capable of forming “embryoid bodies” (EB) comprised of three embryonic germ layers. In this study, hESCs were grown in suspension to induce their differentiation into EBs. Formation of in vitro EB required special cocktail of supplements and growth factors such as glutamine, beta-mercaptoethanol, nonessential amino acids, leukemia inhibitory factor (LIF), and basic fibroblast growth factor (bFGF). Under these conditions, majority of the cells remained in an undifferentiated state. For the formation of EBs, ES cells were transferred using either collagenase or trypsin/EDTA to plastic petri plates to allow aggregation and prevent adherence. About one million ES cells were plated in each of the 50-mm petri plates, and the hEBs were grown in the same culture medium without LIF and bFGF. The differentiation status of the human ES cells and EBs was determined by the expression pattern of several lineage-specific markers such as gamma-globin (hematopoietic cells), alpha-cardiac actin (myocardial cells), neurofilament (neuronal cells), and alpha-fetoprotein (endodermal cells).
Recently, Chen et al. performed a stepwise differentiation of ESCs to insulin-secreting functional beta-cells where ESCs first formed a definitive endoderm in the presence of indolactam-V and FGF, then Pdx1-expressing pancreatic progenitors, followed by the formation of endocrine progenitors and eventually insulin-producing beta-cells (Fig. 2.3) [6, 7].
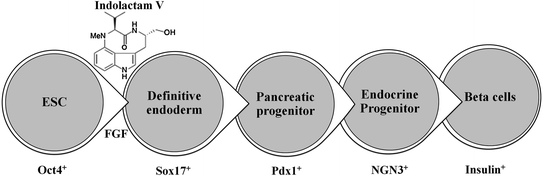

Fig. 2.3
In vitro differentiation of ESCs into insulin-producing beta-cells: Indolactam-V guides the differentiation of ESC into insulin-producing beta-cells via definitive endoderm, pancreatic progenitor, endocrine progenitor, and beta-cells
Thus, strategic development of tissue-specific adult cells with fully functional potential from undifferentiated ES cells is possible and holds great promise for translational application.
Transplantation of hESC-derived cells into human patients:
The first clinical trial using hESC-derived retinal pigment epithelium (RPE) to establish the safety and tolerability in patients suffering from Stargardt’s macular dystrophy and dry age-related macular degeneration was reported by Schwartz et al. [8]. They transplanted a low number of (5 × 104) RPE cells into subretinal space of patient’s eye suffering from different forms of macular degeneration. Preoperative and postoperative ophthalmic examinations such as visual acuity, fluorescein angiography, optical coherence tomography, and visual field testing were performed for its validation (clinicaltrials.gov #NCT01345006 and #NCT01344993). This pilot study generated enthusiasm and hope for cell therapy trials in humans with ESCs, which was sidelined due to the adverse effect of generation of teratoma and ethical regulation. Since isolation of ESCs requires creation, treatment, and destruction of human embryos, hESC research always faces criticism and tight ethical regulation. The effort then moved toward using of adult stem cells, which in spite of limited differential potential has turned out to be a great source for cell therapy application.
2.2.2 Adult Stem Cells
Each adult organ in human body harbors a small population of stem cells that have the ability to maintain tissue homeostasis. These “adult stem cells” remain in quiescent or nondividing state until activated by any injury or disease, and they have limited ability to differentiate into organ/tissue-specific lineages. The common ones are HSCs, mesenchymal stem cells (MSCs), neuronal stem cells, umbilical stem cells, cardiac stem cells, retinal stem cells, and limbal stem cells that reside in their respective tissues. Unlike ES cells, adult stem cells do not require a feeder layer or supporting cells for their growth and thus easier to be engineered using different media, growth factors, and small molecules. They also do not pose a risk for developing teratoma and thus preferable in regenerative medicine and stem cell therapy. However, immune rejections of adult stem cells pose serious challenge in certain cases.
2.2.2.1 Hematopoietic Stem Cells
Pioneering studies in engineering of HSCs started in early 1990s at National Institute of Health for the treatment of patients suffering from adenosine deaminase (ADA) deficiency. These patients were treated with genetically modified CD34+ hematopoietic progenitors using retroviral vectors carrying different transgenes. Out of four successfully treated patients suffering from SCID, three continued doing well up to 3.6 years after gene therapy, whereas one patient suffered serious adverse effect. During a routine checkup after 30 months of gene therapy, lymphocytosis consisting of a monoclonal population of  9/V
9/V 1,
1, 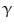 /
/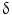 T cells of mature phenotype was detected. One pro-viral integration site was found on chromosome 11 within the LMO-2 locus. This insertion leads to an aberrant expression of the LMO-2 transcript in the monoclonal T-cell population (characteristics of acute lymphoblastic leukemia) [9].
T cells of mature phenotype was detected. One pro-viral integration site was found on chromosome 11 within the LMO-2 locus. This insertion leads to an aberrant expression of the LMO-2 transcript in the monoclonal T-cell population (characteristics of acute lymphoblastic leukemia) [9].
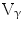 9/V
9/V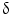 1,
1, 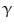 /
/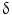 T cells of mature phenotype was detected. One pro-viral integration site was found on chromosome 11 within the LMO-2 locus. This insertion leads to an aberrant expression of the LMO-2 transcript in the monoclonal T-cell population (characteristics of acute lymphoblastic leukemia) [9].
T cells of mature phenotype was detected. One pro-viral integration site was found on chromosome 11 within the LMO-2 locus. This insertion leads to an aberrant expression of the LMO-2 transcript in the monoclonal T-cell population (characteristics of acute lymphoblastic leukemia) [9].These adverse events have resulted in discontinuation of the use of such long terminal repeat (LTR)-driven gamma-retroviral vectors for the genetic manipulations of HSCs, but at the same time, it provided a major thrust for developing novel approaches. New and modified types of retroviral vector such as a “self-inactivating” (SIN) vector [10], lentiviral vectors [11], and lineage-restricted vectors [12] are now entering the clinic. These vectors might reduce the risk of transactivation of proto oncogenes after semi random integrations.
Thrombocytopenia, a deficiency in blood platelets, is a major consequence of several hematological malignancies and chemotherapy [13]. In vitro platelet production from hematopoietic stem and progenitor cells (HSPCs)-derived megakaryocytes (Mks) could augment the supply and elude problems associated with bacterial and viral contamination, as well as immune rejection. Panuganti et al. [14] using HSPCs developed a three-stage strategy for ex vivo expansion of high-ploidy megakaryocytic cells for large-scale platelet production (Fig. 2.4). The CD34+ HSPCs culture was started in a cytokine cocktail at 5 % O2 (pH 7.2). At day 5, cells were shifted to 20 % O2 (pH 7.4) and maintained in 1 of the 17 cytokine cocktails (identified using a 24 factorial design of experiment method to evaluate the effects of interleukin (IL)-3, IL-6, IL-9, and high- or low-dose stem cell factor (SCF) in conjunction with thrombopoietin (Tpo) and IL-11) for expansion of mature Mks from progenitors. The combination of Tpo, high-dose SCF, IL-3, IL-9, and IL-11 produced maximum Mk expansion. These Mks when cultured in IMDM + 20 % BIT 9,500 gave rise to platelets with functional activity similar to that of fresh platelets from normal donors, as validated by basal tubulin distribution and the expression of surface markers.


Fig. 2.4
Production of platelet forming MK cells from HSCs: Schematic representation of step-by-step differentiation of HSCs into MK progenitor, mature MK, polyploid MK, and proplatelet forming MK under a series of cytokine cocktails with increasing pH and pO2
Later Eric Lagasse showed in vivo differentiation of purified HSCs into hepatocytes in a mouse model of a lethal hereditary liver disease. As few as 50 adult HSCs injected intravenously had the capacity to reconstitute hematopoiesis and produce hepatocytes [15].
2.2.2.2 Mesenchymal Stem Cells
MSCs comprised of the major portion of adult stem cells were first identified by Friedenstein from adult bone marrow [16]. These MSCs were shown to differentiate into osteoblasts, chondrocytes, adipocytes, and hematopoietic supporting stroma when a single colony-forming unit-fibroblast (CFU-F) was transplanted in vivo [17].
MSCs can easily be cultured on petri dishes, and their lineages are determined by specific cell markers or enzyme assays. Researchers have shown that MSC can differentiate toward adipocytes or osteocytes in vitro when cultured in adipogenic or osteogenic induction media. During adipogenic induction, the differentiated adipocyte cells stain oil red O-positive indicating a lipid-laden adipocyte phenotype. Similarly, differentiated osteocytes show calcification when stained with alizarin red for calcium deposits [18]. However, determination of the lineage identity in vivo is quite challenging and active research is going on to identify lineage/differentiation-specific biomarkers. A tabulated form of lineage-specific differentiation of MSCs is shown in Fig. 2.5. MSCs can be isolated during routine surgical procedures such as tooth extraction, baby delivery (from placenta and cord blood), or through some special isolation techniques from adipose tissues or bone marrow. These cells can be directed toward lineage-specific differentiation even toward bone using synthetic or natural scaffolds to attain the proper three-dimensional structures. A wide variety of natural and synthetic materials are being tested as scaffolds for bone regeneration. Natural proteins such as collagen [19–21], fibrin [22], silk [23–25], and polysaccharides such as hyaluronic acid and chitosan [26–29] are optimal choices as bone scaffolds. These materials have the advantages of biocompatibility and biodegradability with limited toxicity and may be molded to maintain mechanical flexibility of human bones [22]. Recently, Hassani et al. showed the potential of human endometrial stem cells (EnSCs) to form urinary bladder epithelial cells (urothelium) on nanofibrous silk–collagen scaffolds for construction of the urinary bladder wall [30].
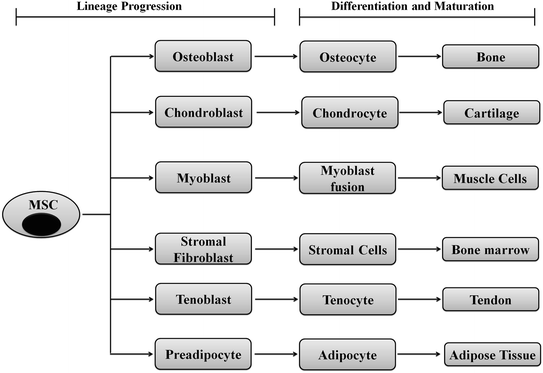

Fig. 2.5
Schematic representation showing differentiation of MSCs to various lineages: MSCs are like gold mines for the regenerative medicine since they could be directed toward any of the cell types through commitment, lineage progression, differentiation, and maturation. A single MSC can differentiate into bone, cartilage, muscle, bone marrow, tendon/ligament, adipose tissue, and connective tissue using appropriate developmental cues
A major thrust area for stem cell therapy is acute myocardial infarction where disruptions of blood supply to the heart muscle cells lead to myocardial infarction or death of cardiomyocytes. Attempts to use stem cells to reduce infarct size and enhance cardiac function in animal models and patients have been exponentially increased in the last decade [31]. Bone marrow and fat tissues serve as the major source of MSCs for cardiovascular disease [32]. Differentiation of mouse BM-MSC into myogenic lineage in vitro has been reported using culture medium supplemented with 5-azacytadine at a concentration of 3 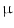 mol/L for 24 h [33]. The purified hMSCs from adult bone marrow engrafted in the myocardium appeared to differentiate into cardiomyocytes. The persistence of the engrafted hMSCs and their in situ differentiation in the small animal models paved the way to use these adult stem cells for human cellular cardiomyoplasty [34].
mol/L for 24 h [33]. The purified hMSCs from adult bone marrow engrafted in the myocardium appeared to differentiate into cardiomyocytes. The persistence of the engrafted hMSCs and their in situ differentiation in the small animal models paved the way to use these adult stem cells for human cellular cardiomyoplasty [34].
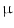 mol/L for 24 h [33]. The purified hMSCs from adult bone marrow engrafted in the myocardium appeared to differentiate into cardiomyocytes. The persistence of the engrafted hMSCs and their in situ differentiation in the small animal models paved the way to use these adult stem cells for human cellular cardiomyoplasty [34].
mol/L for 24 h [33]. The purified hMSCs from adult bone marrow engrafted in the myocardium appeared to differentiate into cardiomyocytes. The persistence of the engrafted hMSCs and their in situ differentiation in the small animal models paved the way to use these adult stem cells for human cellular cardiomyoplasty [34].The enormous potential of various adult stem cells in curing diverse diseases encouraged researchers to explore their potential systematically either in vitro or in vivo condition. Some of these examples on MSC-based applications are mentioned in Table 2.1.
Table 2.1
In vitro differentiation of adult stem cells to their respective lineages using small molecules, appropriate scaffold stiffness, cocktail of drugs and culture media
Cell type | Culture condition | Lineage | References |
|---|---|---|---|
hESC | Ascorbic acid | Cardiac myocytes | [35] |
BM-MSCs | Ascorbic acid, BMP-2, dexamethasone, TGF-beta, and insulin | Osteogenic | [36] |
DMEM/10 % FBS + 0.5 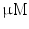 dexamethasone, 0.5 mM isobutyl-l-methylxanthine, and 10 dexamethasone, 0.5 mM isobutyl-l-methylxanthine, and 10 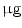 /ml insulin /ml insulin | Adipogenic | [37] | |
DMEM/10 % FBS, 100 nM dexamethasone, 10 mM 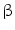 -glycerophosphate, and 50 -glycerophosphate, and 50 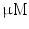 ascorbic acid ascorbic acid | Chondrogenic | [37] | |
Laminin-1 without serum and differentiation growth factors, valproic acid, insulin, and butylated hydroxyanisole | Neurogenic | ||
Cardiac MSC | (5 % FBS) in 5 mM all-trans retinoic acid, 5 mM phenyl butyrate, and 200 mM diethylenetriamine/nitric oxide Transferrin, IL3, IL6, and VEGF | Myogenic | |
MSC | 1. Osteogenic differentiation on stiffness (45–49 kPa) 2. Myogenic differentiation (13–17 kPa) | Osteogenic Myogenic | [40] |
Limbal stem cell | Human amniotic membrane + human corneal epithelial cell medium + autologous serum | Limbal epithelium | [41] |
2.2.3 Induced Pluripotent Stem Cell
While stem cell therapy is emerging as a promising alternative for diseases and genetic disorders where drugs or gene therapy fail, it is limited by availability and stringent culture conditions. Exploiting epigenetic influence on phenotypic outcome, researchers have developed powerful genetic platforms for reversal of differentiated adult cells back to an embryonic state. Such reprogrammed cells are known as “induced pluripotent stem cells (iPSCs),” and the reprogramming strategies include “therapeutic cloning” and “nuclear reprogramming.” Both these strategies act through ectopic introduction of a small number of pluripotency-associated transcription factors into differentiated tissue-specific cells. iPSCs have the ability to differentiate into any of the three germ layers, ectoderm, mesoderm, and endoderm and the respective lineage-specific fully differentiated and functional cells/tissues.
The concept of iPSCs demonstrated long back by Sir John Gurdon when he successfully cloned a frog using intact nuclei from intestinal epithelium cells of [42]. Later, he showed that even nuclei from terminally differentiated adult cells (e.g., blood cells, skeletal muscle, and kidney cells) could generate Xenopus larvae with nuclear transfer [42]. Decades later, in 2006, Takahashi et al. demonstrated the ability of adult mouse fibroblasts to reprogram themselves into pluripotent stem cells by introduction of four key transcription factors (Oct3/4, Sox2, c-Myc, and Klf4) [43]. In November 2007, two independent studies were published simultaneously on successful transformation of differentiated human cells into pluripotent stem cells. While Takahashi et al. used retroviral delivery Oct3/4, Sox2, Klf4, and c-Myc combinations to induce pluripotency in human fibroblasts, Yu et al. delivered Oct4, Nanog, Sox2, and LIN28 by lentiviral transduction in hESC-derived mesenchymal cells to induce pluripotency [44, 45]. These groundbreaking experiments by Sir Gurdon and Yamanaka and his group were acknowledged by Nobel Prize award in 2010.
The most exciting and oversimplified part of iPSC generation is that a combination of only four transcription factors is able to reverse the differentiation process. To identify this main core of pluripotent factors, Yamanaka et al. (2006) evaluated twenty-four candidate genes and Thomson et al. (2007) screened sixteen transcription factors in an assay system in which the induction of the pluripotent state could be detected through the development of resistance to neomycin gene. Both the groups identified Oct4, Sox2, and Nanog as the major pluripotency determinants. Though promising, the current iPSC reprogramming method experiences certain drawbacks such as
1.
Requires host cell to be genetically engineered to express a drug resistance gene driven by a marker of pluripotency.
2.
Requires viral-mediated integration of transgenes into the genome.
3.
Reactivation of c-Myc in differentiated progeny of the induced ES-like cells is common and may result in tumor formation [46].
Thus, alternative approaches such as use of purified transcription factors, replacement of c-Myc, and strategy to avoid drug resistance selection method are being explored [47].
Their et al. [48] reported generation of TAT-modified cell permeate versions of recombinant Oct4 and Sox2 proteins (Oct4 TAT and Sox2 TAT), and later, Zhou et al. generated protein-piPSCs from murine embryonic fibroblasts. The deleterious effects of c-Myc could be circumvented by using n–myc, and host cell need not be drug resistant if using serum-free condition for iPSCs generation [49]. Recently, miRNA particularly the miR302/367 cluster was used to generate iPSCs from mouse and human somatic cells without adding the exogenous transcription factors. This miRNA-based reprogramming was found to be more efficient (twofold) than the standard Oct4/Sox2/Klf4/Myc-mediated reprogramming and ultimately overcome the deleterious effect of c-Myc reactivation [50].
Using the above-mentioned methods, one can now generate individual-specific iPS cell lines to derive patient-specific progenitor cells and eliminate immune rejection crisis. Moreover, iPSC-based technology will facilitate the production of cell line panels that closely reflect the genetic diversity of a population enabling the discovery, development, and validation of therapies tailored for each individual. Till today, iPSCs has been generated from ten different species mouse, human, rhesus monkey, rat, dog, rabbit, horse, and bird [43, 44, 51–58] and into various lineages as listed in Table 2.2.
Table 2.2
In vitro differentiations of species-specific iPSCs toward various lineages in their respective culture condition
iPSCs | Culture condition | Lineage | Species | Reference |
|---|---|---|---|---|
Murine | Valproic acid, zonisamide, and estradiol | Neural | Rat model of ALS | [59] |
ES medium without LIF for the first 4 days and day 5 onwards in differentiating medium supplemented with RPE-conditioned medium | RPE | Mouse | [60] | |
Human | Serum-free embryoid-body-like aggregates | Dopaminergic neurons | Mouse | [61] |
Keratinocyte growth factor and fibroblast growth factor | Hepatocyte-like cell | Human | ||
Embryoid bodies | Erythropoietin | Human | [64] | |
EGF and bFGF | Oligodendrocyte progenitors | Rat model | [65] | |
Porcine | Activin A, bFGF, BMP-4, and oncostatin | Hepatic | Porcine | [66] |
However, it still would be a long way for iPSCs to reach the clinic, which requires stringent and systematic validation of lineage-specific differentiation.
2.3 Monitoring of Stem Cells
Success of regenerative medicine and stem cell therapy depends on efficient in vivo differentiation of stem cells into specific lineages. Monitoring of engineered stem cells in cell cultures and in vivo before and after transplantation is a prerequisite for any stem cell application. It is also necessary to perform such studies directly in living subjects in a longitudinal, reliable, and accurate manner. Various microscopic techniques are extensively used for detail visualization of growth, differentiation, and functional validation of stem cells and iPSCs in cultures. Some of these techniques are also utilized for monitoring of the stem cells in living subjects with high resolution. In parallel, macroscopic or noninvasive in vivo imaging modalities turn out to be indispensible for longitudinal monitoring of translational applications. Commonly used noninvasive imaging modalities for stem cell therapy are radioisotopic imaging (PET or positron emission tomography and SPECT or single-photon emission computed tomography), CT, ultrasound, magnetic resonance imaging (MRI), and optical imaging (bioluminescence and fluorescence). The next two sections will elaborate microscopic and macroscopic imaging of stem cells, an essential requirement for clinical application (Fig. 2.6).
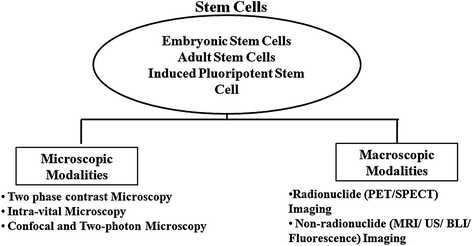

Fig. 2.6
Imaging modalities for in vitro and in vivo monitoring of stem cells: Naive or engineered stem cells in in vitro culture or transplanted in living subjects can be visualized noninvasively either by microscopic or by macroscopic imaging techniques. Microscopic modalities include phase contrast microscopy, intravital microscopy, confocal and two-photon microscopy, whereas macroscopic modalities include radionuclide-based (positron emission tomography or PET and single-photon emission computed tomography or SPECT) and nonradionuclide-based (MRI, ultrasonography imaging, bioluminescence, and fluorescence) imaging
2.3.1 Microscopic Techniques
For centuries, microscopy is an indispensable tool for visualizing dynamics of biomolecules in live cells. Optical microscopic techniques including conventional light (phase contrast) microscopy, fluorescence microscopy, confocal and multiphoton microscopy, intravital microscopy (IVM) have emerged as powerful tools for noninvasive monitoring and characterization of engineered stem cells and tissues [67].
2.3.1.1 Phase Contrast Microscopy
The age-old phase contrast microscopy is routinely required to monitor the culture conditions and kinetics of HSCs during their expansion for therapeutic use. Recently, these microscopes were improved with automated time-lapse system to capture the mitotic divisions of stem cells such as multipotent C3H10T1/2 mesenchymal and C2C12 myoblastic stem cells in real time [68, 69].
2.3.1.2 Fluorescence Microscopy
The complexity of the biological samples can be unraveled by labeling specimen with a fluorophore to achieve single-cell resolution when monitored with fluorescent microscopes. Stem cell labeling for fluorescence microscopy includes DNA binding dyes (such as Hoechst dye, BrdU, DAPI), nanoparticles and quantum dots, the later ones are also suitable for in vivo imaging. These labeling methods can only be used for short-term cell tracking due to loss of fluorophores through cell division. For long-term monitoring, tracking, engraftment and differentiation of stem cells, genetic manipulation with fluorescent proteins of different excitation and emission spectra (GFP and RFP and their mutants) is an ideal approach. These proteins can also be coupled to another protein to act as molecular reporters in living cells [70].
Tumbar et al. [71] developed a new strategy to identify multipotent epithelial stem cells (ESC) in their native environment by fluorescent labeling. These quiescent cells residing in the bulge of hair follicles can differentiate into various cell types upon stimulation. Transgenic mice expressing a H2B-GFP fusion protein under tetracycline-regulated keratinocyte-specific K5 promoter showed specific GFP expression only in rapidly dividing skin epithelium. Administration of doxycycline led to the loss of GFP expression in the keratinocytes but not in the SCs due to their slow cycling and quiescent nature. The fluorescence microscopy of the sections from the keratinocytes clearly demonstrated the label-retaining capability of SC niche. This method was later used by many research groups to isolate and purify the label-retaining cells (LRCs) or SCs for further characterization [70, 71].
Fluorescent probes with emission wavelengths in the near-infrared (NIR) spectra (~700–800 nm) enhance the feasibility of tracking cells in vitro and in small animal models due to high depth penetration and lower absorption and scattering [72]. Several dyes including NIR fluorochrome DiD, a lipophilic dye that binds to the cell membrane, have proven effective for both in vitro labeling of MSCs and in vivo cell tracking with optical imaging [73, 74].
As described in Sect. 2.2.3, direct reprogramming of somatic cells into iPSCs can be achieved by overexpression of four reprogramming factors (RFs). A dynamic fluorescence microscopy study of iPSCs expressing Nanog-GFP suggests that the number of cell divisions is a key parameter of driving epigenetic reprogramming to pluripotency [75]. Similarly, fluorescence microscopy combined with long-term time-lapse imaging and single-cell tracking revealed the “birth” of pluripotent cells and early iPSC clusters from murine embryonic fibroblasts transduced with multicistronic lentiviral vectors carrying RFs (Klf4, Sox2, Oct4, Myc) tagged with different fluorescence proteins (GFP, RFP, YFP) [76].
2.3.1.3 Confocal Microscopy and Multiphoton Microscopy
A major drawback of fluorescence microscopy is that irrespective of the vertical focusing of the specimen, illumination causes the entire specimen to fluoresce and is unable to produce tomographic images. Confocal microscopy, multiphoton microscopy, and intravital microscopy are competent of generating tomographic imaging essential for localizing fluorescent targets in three-dimensional space [77–79].
In a recent study, transgenic ES cells co-expressing myristoylated RFP (labels plasma membrane) and histone H2B-GFP (labels active chromatin) fusions were introduced into a nontransgenic embryo and then dissected out of the maternal uterus at mid-gestation period and cultured ex utero on the stage of a confocal microscope. These labeled ES cells produced information on dynamic changes in morphology and chromatin distribution that occurred during mitotic progression [80]. The powerful confocal/two-photon hybrid microscopy can also track the clonal history of HSPCs expressing various fluorescence proteins noninvasively in intact tissues, including bone marrow with long-term monitoring after transplantation [81].
2.3.1.4 Intravital Microscopy
Intravital microscope (IVM) can be referred as “microscope for living subjects,” which enables single-cell imaging in thin sections of live tissues [82]. In a remarkable study, Rompolas et al. [83] monitored the regeneration of hair follicles temporarily in a transgenic mouse expressing H2B-GFP driven by keratin 14 promoter. They demonstrated that stem cells are quiescent during initial stages of hair regeneration and their progeny is actively dividing within follicular organization [83].
In another elegant study, Takayama et al. [84, 85] imaged the functioning of human iPSC-derived platelets during thrombus formation by intravital microscopy in live mice. The study demonstrated that transient expression of c-Myc was critical for efficient platelet generation from human iPSCs, which were capable of mediating hemostasis and thrombosis in a laser-induced vessel wall injury.
2.3.2 Macroscopic Imaging Modalities
Though microscopic imaging techniques can generate critical information, they are restricted at cellular level and do not depict the kinetics, distribution, and location of in vivo differentiation of stem cells in a living subject. Even IVM requires an artificially created “window chamber” or “tissue flap” at the body surface and is not applicable for deep tissue imaging. Recent developments in macroscopic or in vivo imaging modalities enable the “visualization, characterization, and measurement of biological processes at the molecular and cellular levels in humans and other living systems.” Along with other applications, these modalities are now widely used for imaging stem cell delivery, migration and localization, cell viability, and therapeutic effects in various diseases [86–88].
The Six commonly used modalities for imaging stem cell therapy are ultrasound, CT, SPECT, PET, MRI, and optical. For many instances, multimodality approaches that can collectively assess different parameters specific for each modality are gaining popularity. For imaging the stem cells in vivo, it is required to label the cells with an appropriate probe. The two important methods for labeling cells are as follows:
(a)
Direct labeling such as with magnetic resonance contrast agents, radionuclides, fluorophores, and nanoparticles.
(b)
Indirect labeling with reporter genes.
Direct labeling of cells poses limitation to long-term monitoring since the signal gets diminished by dilution or loss of labeling agents via cell division or differentiation. This limitation can be overcome with “indirect-labeling strategy” where cells are exogenously labeled with reporter genes (bioluminescence, fluorescence, PET, SPECT, or MR reporters) and then implanted for imaging. Indirect labeling requires genetic manipulation of the cells that are often not possible to follow in clinics [86–90]. However, both the strategies have own advantages and disadvantages and should be implemented by experimental need.
2.3.2.1 Radionuclide Imaging
Majority of the radionuclide imaging techniques follow direct-labeling strategies and are extensively used in human studies. Both radionuclide imaging techniques, i.e., PET and SPECT, have picomolar sensitivity and high tissue penetration with least attenuation and are tomographic in nature.
Positron Emission Tomography (PET)
In PET, two 180° apart high-energy (511 keV) gamma rays produced by the annihilation of a positron (from the radioactive atom) with a neighboring tissue electron are captured by detectors and produce a three-dimensional image of functional processes in living subjects. The commonly used positron-emitting isotopes are 11C, 13N, 15O, 124I, 64Cu, and 18F. The widely used PET tracers in clinic are 2-deoxy-2-18F fluoro-d-glucose (18F-FDG, which images glucose metabolism) and 3-deoxy-3-18F fluorothymidine (18F-FLT, which images cell proliferation). Several studies have elaborated the use of PET imaging approach for the stem cells therapy in various human disorders [91–100] (Table 2.3). Some studies have also used small animal models for studying the role of human stem cells in homing, engraftment, and survival through PET imaging [101–105].
Table 2.3
Stem cell therapy studies using PET imaging approach in human
Stem cells | Radionuclides | Applications | Reference |
|---|---|---|---|
HSCs | 18F-FDG | Homing and tissue distribution of intracoronary injected peripheral HSCs | [106] |
MSCs | 18F-FDG | Functional efficacy of MSC therapy in patients with multiple system atrophy | [107] |
Cord blood stem cells (CBSC) | 18F-FDG | CBSC transplant in patients for local engraftment and reconstitution of haematopoiesis | [108] |
CD33 + bone marrow stem cells | 13N-ammonia and 18F-FDG | Improvement in the myocardial perfusion in patients AMI | [109] |
HSCs | 18F-FDG | HSC therapy for osteosarcoma | [110] |
Herpes simplex virus type 1 thymidine kinase (HSV-tk) is the most widely used reporter gene for PET imaging for preclinical as well as clinical studies [90]. 18Fluorine-labeled FIAU (2′-fluoro-2′-deoxy-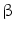 –D-arabinofuranosyl-5-iodouracil) and FHBG (9-(4-fluoro-3-hydroxy-methyl-butyl) guanine) are the two common reporter probes used for HSV1-tk. This reporter gene–reporter probe approach has been used to monitor viability of stem cells after transplantation in myocardium, tracking, and survival of autologous MSCs in pig myocardium and tumor stroma [111, 112].
–D-arabinofuranosyl-5-iodouracil) and FHBG (9-(4-fluoro-3-hydroxy-methyl-butyl) guanine) are the two common reporter probes used for HSV1-tk. This reporter gene–reporter probe approach has been used to monitor viability of stem cells after transplantation in myocardium, tracking, and survival of autologous MSCs in pig myocardium and tumor stroma [111, 112].
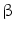 –D-arabinofuranosyl-5-iodouracil) and FHBG (9-(4-fluoro-3-hydroxy-methyl-butyl) guanine) are the two common reporter probes used for HSV1-tk. This reporter gene–reporter probe approach has been used to monitor viability of stem cells after transplantation in myocardium, tracking, and survival of autologous MSCs in pig myocardium and tumor stroma [111, 112].
–D-arabinofuranosyl-5-iodouracil) and FHBG (9-(4-fluoro-3-hydroxy-methyl-butyl) guanine) are the two common reporter probes used for HSV1-tk. This reporter gene–reporter probe approach has been used to monitor viability of stem cells after transplantation in myocardium, tracking, and survival of autologous MSCs in pig myocardium and tumor stroma [111, 112].Recently, human sodium iodide symporter (hNIS) gene has emerged as an important PET reporter gene that could be used for SPECT imaging as well. Uptake of 124Iodine was seen in areas deficit of myocardial perfusion in rat myocardium injected with MSCs expressing hNIS gene by PET imaging [113]. NIS-mediated 124I PET imaging was also used to monitor the delivery and survival of endothelial progenitor cells (EPCs) after transplantation into the rat heart [114]. Some other promising PET reporter genes for stem cell therapy are dopamine 2-like receptor (D2R), human somatostatin receptor subtype 2 (hSSTr2), human norepinephrine transporter (hNET), neurotensin receptors, and cytosine deaminase [115].
Stem cell therapy is often benefited from multimodality imaging approaches. In an elegant study, Cao et al. [116] monitored the survival, proliferation, and migration of ESCs expressing a triple fusion (TF) reporter comprised of a fluorescence (mrfp), a bioluminescence (fluc), and a PET reporter (ttk) gene after transplantation into rat myocardium for 40 days (Fig. 2.7). Some studies have used bifusion reporters such as tk-GFP to monitor neuronal stem cell, therapy in treatment of malignant glioma [117].
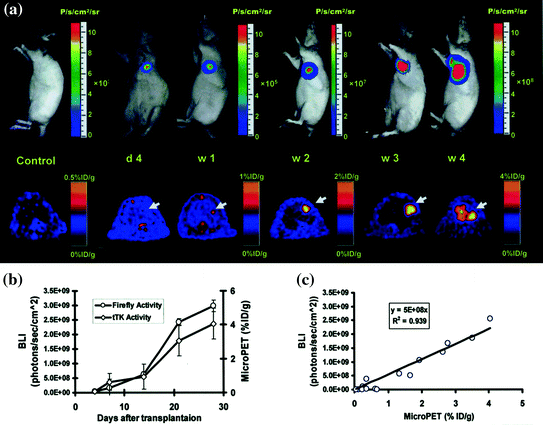

Fig. 2.7
Multimodality imaging for long-term monitoring of mouse embryonic stem cells expressing a triple fusion reporter (ES-TF): a Representative images of animal injected with ES-TF in infracted rat heart showed significant increase in bioluminescence (top) and PET (bottom) signals from day 4, week 1, week 2, week 3, and week 4. b Quantification of imaging signals showed a drastic increase in luciferase and thymidine kinase activities from week 2 to week 4. c Quantification of cell signals showed a robust in vivo correlation between bioluminescence and PET imaging (r 2 = 0.92). (Reprinted from Cao et al. [116] with permission)
Single-Photon Emission Computed Tomography (SPECT)
In contrast to PET imaging, SPECT imaging is a log-order less sensitive technique due to the presence of collimators (to restrict detection of nonspecific random gamma rays) between the detectors. The widely used SPECT radionuclides are 99mTc, 111In, and 123I. SPECT imaging probes are extensively applied to image in vivo trafficking and biodistribution of MSCs in myocardial injuries in large animal models [118–120]. Some human clinical studies have also used 111In-oxine to track bone marrow stem cells or pro-angiogenic progenitor cells in acute and chronic myocardial injuries. Goussetis et al. [121] performed SPECT imaging with 99mTc hexamethylpropyleneamine-oxime-labeled autologous CD133−CD34+ bone marrow progenitor cells transplanted in patients with ischemic cardiomyopathy. Higher uptake of radioactivity was observed in the infracted area of the heart, suggesting preferential migration and retention of stem cells in the chronic ischemic myocardium.
Both HSV1-tk and hNIS genes can serve as SPECT reporter genes in combination with probes labeled with SPECT isotopes (123I/125I-labeled probes FIAU or 99mTc) [113, 122]. Table 2.4 summarizes various studies involved in stem cell visualization by SPECT in human. Studies related to small animal models can be found in other articles [123–125].
Table 2.4
Stem cell therapy studies using SPECT imaging approach in human
Stem cell types | Radionuclides | Applications | Reference |
|---|---|---|---|
MSCs | 111In-tropolone | Delivery, tracking, and differentiation of stem cells | [126] |
BM-MSCs | 111In-oxide | Monitoring blood flow and bone metabolism after cell transplantation | [127] |
CD34+ stem cells | 99mTc hexamethylpropyleneamine oxime | 5-year follow-up of CD34+ stem cell in nonischemic cardiomyopathy patients | [128] |
MSCs | 99mTc-pertechnetate | Demonstrate the feasibility of MSCs as virus carriers to ovarian tumors in phase I clinical trails | [129] |
BM-MSCs | 99mTc-sestamibi | Improvement in cardiac function after BMMSCs transplantation in patients with acute myocardial infarction | [130] |
Autologous CD34+ cells | 99mTc-exametazime | Homing of transplanted cells to injured myocardium | [131] |
BM-HSCs | 99mTc | Repair efficacy for ischemic heart | [132] |
2.3.2.2 Optical Imaging
Optical imaging has emerged as an established tool to assess efficacy and treatment outcomes for cell-based therapeutics in preclinical models. Two optical imaging methods, bioluminescence, and fluorescence are extensively used in stem cell research.
Fluorescence Imaging (FLI)
Fluorescence imaging is the only modality that directly translates the finding/observation from live cell to live animals. However, due to inherent autofluorescence and signal attenuation issues, whole-body FLI has not been extensively used for stem cell therapy. Tzukerman et al. [133] applied fluorescence imaging to demonstrate the growth and invasiveness of cancer cells in the niche of teratomas derived from hESCs. Tao et al. [134] reported that e-GFP is a better probe than DS-Red protein for long-term monitoring of HSCs.
Other than reporter gene strategy, FLI can be performed with exogenous contrast agents such as quantum dots, nanoparticles, and fluorescent dyes that emit light in the visible, red, and near-infrared region. Inorganic fluorescent semiconductor nanocrystals (quantum dots, QDs) are rapidly replacing organic fluorophores like indocyanine green with NIR emission due to their ability for multiplex imaging [135]. Lin et al. [136] for the first time demonstrated the in vivo multiplex imaging of mouse ES cells labeled with six quantum dots (QDs). The six emission spectra from all the six QDs were recorded with a single excitation light source. These QDs are emerging as a promising tool for tracking stem cells within deep tissues noninvasively in vivo. Human ESC-derived cardiomyocytes (hESCs-CM) were also labeled with indocyanine green for noninvasive tracking by fluorescent imaging [137].
Bioluminescence Imaging (BLI)
Among all the functional imaging modalities, BLI acts only through reporter gene–reporter probe strategy and has been extensively used for monitoring stem cell therapy. Though restricted to small animals, bioluminescence imaging generates essential clues on differentiation, behavior, and viability of stem cells after transplanted in small animals, assisting to predict the behavior of stem cell therapy in humans. A glimpse of the large number of BLI-based studies is summarized in Table 2.5, and a few important studies are discussed.
Table 2.5
Studies involving BLI approach for stem cell therapy
Stem cell types | Origin | Applications | Reference |
|---|---|---|---|
HSCs | Human | Longitudinal monitoring of human HSC engraftment | [140] |
Neural progenitor cells | Murine | Migratory capability of NPCs and their preferential accumulation in brain tumors on CNS | [141] |
Embryonic rat H9C2 cardiomyoblast | Rat | Location, magnitude, and survival duration of embryonic cardiomyoblast | [142] |
ESC-derived insulin-producing cells (IPCs) | Murine | Novel source of unlimited cells for transplantation to treat type 1 | [143] |
ESCs | Murine | Longitudinal monitoring and tumorigenic potential of ESCs | [144] |
Human | Longitudinal monitoring of differentiation in the tumors | [145] | |
Human | Preferential differentiation of hESC-derived CD34+ cells into endothelial cells | [146] | |
Mouse | Longitudinal monitoring of implanted ESCs in rat corpus cavernosum | [147] | |
Human | Serial imaging of human embryonic stem cell engraftment and teratoma formation in murine model | [122] | |
Neural stem cells | Mouse | Improved engraftment of neural stem cells | [148] |
MSCs | Mouse | Migration and engraftment of transplanted cell into primary breast tumor sites | [149] |
Monitoring survival of transplanted MSCs injected intramyocardially | [150] | ||
Homing to kidneys in mice with ischemia- and reperfusion-induced acute kidney injury (AKI) | [151] | ||
MSCs expressing bone morphogenetic protein 2 (BMP2) | Human | Bone and cartilage repair in articular fractures | [152] |
Umbilical cord blood HSCs | Human | Cell engraftment of HSCs after bone marrow transplantation in nonobese diabetic/SCID mice | [153] |
BM-MSCs | Human | Monitoring inhibition and eradication of glioma with BM-MSCs labeled with Delta-24-RGD | [154] |
MSCs | Murine | Localization, survival, proliferation, and differentiation of MSCs to osteoblasts and adipocytes | [155] |
iPSCs | Human and Murine | Long-term tracking of iPSCs in the gastrocnemius muscle of recipient mice monitored | [156] |
ESCs-derived cardiomyocytes (CM) | Murine | Tracking of immature (ESCs-CM) demonstrate longer survival than the mature CM | [157] |
Tsuji et al. [138], using iPSC-derived neurospheres in a mouse model of spinal cord injury, demonstrated that iPSCs can “safely” promote locomotor function recovery in injured mouse models with BLI. They observed that these cells can even differentiate into trilineage neural cells in the injured spinal cord. In another study, Daadi et al. [139] investigated the efficacy of human neural stem cells (hNSCs) derived from human ES cells to repair brain injury. In this study, rats with neonatal HI (hypoxic-ischemic) brain injury were implanted with hNSCs expressing luciferase and their survival was monitored using BLI. The study suggests that hNSCs transplants are able to enhance brain injury repair in response to HI brain injury and that the location and survival can be monitored noninvasively. Further, the deleterious effect of ESC differentiation on teratomas was spatially and temporally monitored by Cao et al. [116] with high sensitivity, which was not possible with other imaging modalities (Fig. 2.7).
2.3.2.3 Magnetic Resonance Imaging
MRI is the sole imaging modality that generates both functional information and anatomical information. Among all the other noninvasive imaging techniques, MRI has the highest spatial resolution (~100  ) and thus is the most preferred strategy for stem cell imaging. Since endogenous molecules (such as H2 atoms) do not generate enough contrast to achieve that high resolution, supra-paramagnetic iron oxide (SPIOs) and paramagnetic nanoparticles are often used to label the cells to enhance image contrast. However, SPIO-based MRI is not well suited for long-term monitoring since SPIOs get diluted with cell proliferation and are often engulfed by macrophages [86]. Chelated gadolinium (Gd3+), manganese (Mn2+), and iron (Fe3+) could also act as contrast agents. Recently, certain metal-ion-based enzymes namely metalloproteinase, transferrin, ferritin, tyrosinase are being evaluated as reporter genes in MR imaging [87].
) and thus is the most preferred strategy for stem cell imaging. Since endogenous molecules (such as H2 atoms) do not generate enough contrast to achieve that high resolution, supra-paramagnetic iron oxide (SPIOs) and paramagnetic nanoparticles are often used to label the cells to enhance image contrast. However, SPIO-based MRI is not well suited for long-term monitoring since SPIOs get diluted with cell proliferation and are often engulfed by macrophages [86]. Chelated gadolinium (Gd3+), manganese (Mn2+), and iron (Fe3+) could also act as contrast agents. Recently, certain metal-ion-based enzymes namely metalloproteinase, transferrin, ferritin, tyrosinase are being evaluated as reporter genes in MR imaging [87].
 ) and thus is the most preferred strategy for stem cell imaging. Since endogenous molecules (such as H2 atoms) do not generate enough contrast to achieve that high resolution, supra-paramagnetic iron oxide (SPIOs) and paramagnetic nanoparticles are often used to label the cells to enhance image contrast. However, SPIO-based MRI is not well suited for long-term monitoring since SPIOs get diluted with cell proliferation and are often engulfed by macrophages [86]. Chelated gadolinium (Gd3+), manganese (Mn2+), and iron (Fe3+) could also act as contrast agents. Recently, certain metal-ion-based enzymes namely metalloproteinase, transferrin, ferritin, tyrosinase are being evaluated as reporter genes in MR imaging [87].
) and thus is the most preferred strategy for stem cell imaging. Since endogenous molecules (such as H2 atoms) do not generate enough contrast to achieve that high resolution, supra-paramagnetic iron oxide (SPIOs) and paramagnetic nanoparticles are often used to label the cells to enhance image contrast. However, SPIO-based MRI is not well suited for long-term monitoring since SPIOs get diluted with cell proliferation and are often engulfed by macrophages [86]. Chelated gadolinium (Gd3+), manganese (Mn2+), and iron (Fe3+) could also act as contrast agents. Recently, certain metal-ion-based enzymes namely metalloproteinase, transferrin, ferritin, tyrosinase are being evaluated as reporter genes in MR imaging [87].In contrast to optical imaging where smaller animals such as mouse and rat are preferred as model systems, MR-based stem cell imaging is tested both in smaller and larger animals and in humans. The first autologous transplantation of iron oxide-labeled iPSCs reprogrammed from canine adipose stromal cells and fibroblasts showed repair of infracted myocardium and hindlimb ischemia by MR imaging in adult mongrel dogs [158]. Similarly, an enhanced effect of combining human cardiac stem cells and bone marrow MSCs to reduce infarct size and restore cardiac function after myocardial infarction was followed by MR imaging in a Yorkshire swine model [159].
To overcome the shortcoming of the contrast agents, reporter-gene-based strategies are also being employed in stem cell imaging by MR. Liu et al. [160] showed that engraftment of transgenic mouse ESCs expressing human ferritin heavy chain (FTH) resulted in increased cellular iron uptake and MRI contrast and did not interfere with stem cell pluripotency, neural differentiation, and teratoma formation.
Stem cell therapy has immense potential to treat neurodegenerative diseases, traumatic injury, and stroke. However, risk is associated with intracranial surgery used to deliver the cells to the brain. In some studies, MRI was combined with ultrasound modality to obtain higher sensitivity and resolution. For targeted delivery of neural stem cells to brain, Burgess et al. [161] employed MRI-guided focused ultrasound (MRIgFUS) imaging to monitor noninvasive delivery of stem cells from the blood to the brain by opening the blood–brain barrier at specific regions (striatum and hippocampus) in rat brain. Entry of cells crossing the BBB to brain was verified by MRI. The study also demonstrated that these stem cells started expressing double cortin, a marker of immature neurons, indicating occurrence of in vivo differentiation. An excellent review by Qiu et al. [162] described many such MRI-based studies for stem cell therapy such as migration and homing of hematopoietic stem–progenitor cells to injured arteries and atherosclerosis, stem–progenitor-cell-mediated vascular gene therapy, and several novel techniques for magnetic labeling of stem or progenitor cells. Table 2.6 describes some of the MRI-based stem cell tracking studies in living subjects.
Table 2.6
MRI-based stem cell tracking studies in living subjects
Stem cells | Contrast agents (CAs)/reporter genes | Applications | Reference |
|---|---|---|---|
hESCs | SPIO MnCl2 | Comparison between two CAs to monitor stem cell therapy for failing heart | [163] |
hESCs | SPIO | Long-term monitoring of transplanted cells in mouse myocardial infraction model | [164] |
hESC-derived neural stem cells | SPIO | Long-term monitoring of differentiation in rat brain injury model | [165] |
Rat MSCs | Gadolinium–diethylene triamine penta-acetic acid (Gd-DTPA) | Transplanted cells are used to track spinal cord injury | [166] |
ESCs/MSCs | Iron oxide nanoparticles | Evaluation of migration and fate of transplanted cells in rat central nervous system (CNS) | [167] |
Pig cardiospheres (Cs): clusters of cardiac stem cells | Ferritin | In vivo tracking of stem cells in rat model of myocardial infarction
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|