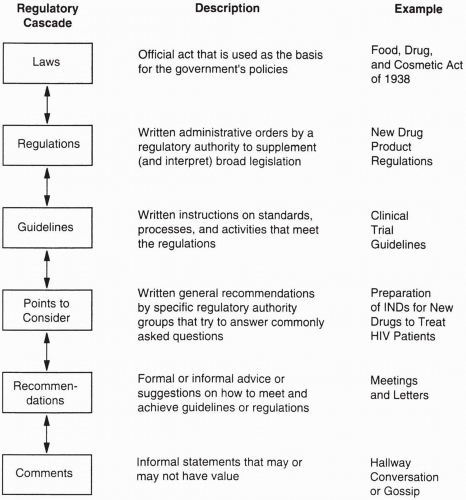
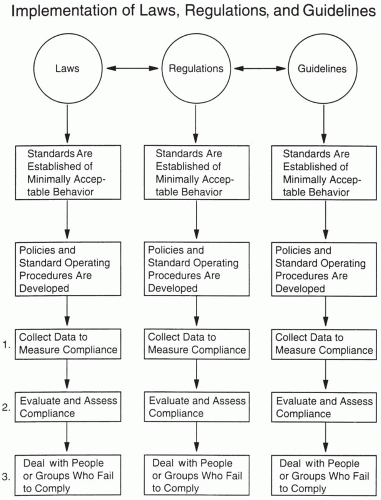
What Are Standards?
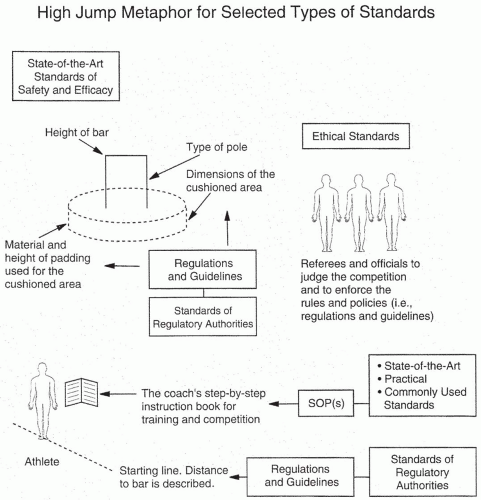
Standards may be thought of as bars on a high jump that an athlete must clear to be successful in a particular endeavor (
Fig. 4.3).
Figure 4.3 also illustrates related metaphors for a few other terms discussed in this chapter. Numerous types of standards exist for the development of new drugs (
Table 4.2). These include (a) ethical standards, (b) regulatory standards, (c) state-of-the-art technical standards, (d) practical standards, and (e) commonly used industry standards. In some cases, all of these standards are nearly identical, but in most situations, there are great differences among them. Adherence to one set of standards often leads to failure to meet others. Moreover, meeting or even surpassing one set of standards (e.g., regulatory) in a particular country (e.g., Sri Lanka) does not mean that (regulatory) standards for the same issue will be met in all countries. Great differences from country to country often exist for all types of standards, except for technical state-of-the-art standards, which are universal.
Why Create Standards?
Ethical standards, practical standards, state-of-the-art technical standards, and commonly used industry standards all exist for many particular aspects of drug development. Regulatory standards are the only type that do not a priori (i.e., necessarily) exist. Regulatory standards are established by a government to protect the health of a nation’s patients when taking drugs. Prior to the 20th century, few regulatory standards existed, and the overall control of drug composition, manufacture, and sale was often grossly inadequate to protect people’s health. Many dangerous drugs were widely promoted and sold for use in totally unethical ways (e.g., morphine was given as a pacifier to quiet “noisy” babies).
How Are Standards for Drug Development Created?
Each of the five general types of standards described in the following text is often applied to specific issues in toxicology, clinical trials, manufacturing, or any other aspect of the discovery, development, production, or marketing of drugs. This chapter cannot explore those detailed applications. Rather, a brief description is given of how each of the types of standards is created.
Regulatory standards are derived from laws that are initially created by governments (e.g., Congress in the United States, Parliaments in many countries) and interpreted and defined when possible through regulations, guidelines, and comments by the national regulatory authority that is charged with approving and reviewing the safety of drugs and determining which drugs may be allowed on the market.
State-of-the-art technical standards are created by innovative scientists and clinicians in the forefront of their fields and by those who influence thinking in the disciplines in which they work. Some of this latter group of people are methodologists who are concerned with refining established approaches and methods within their own discipline. These become known via multiple methods such as endorsement by professional societies, trade associations, or consensus conferences, or by common practice based on published studies.
Practical standards are created by the companies developing drugs. They determine what level of effort is feasible and cost effective to achieve the specific goals they set. Practical standards are often below state-of-the-art standards. Nonetheless, practical standards are often appropriate when studying certain topics or issues.


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access