Introduction to Clinical Trials
The history of drugs has never been a particularly attractive subject in medical education, and one reason for this is that it is so unbelievably deplorable a story. For century after century, all the way into the remote millennia of its origins, medicine got along by sheer guesswork and the crudest sort of empiricism. It is hard to conceive of a less scientific enterprise among human endeavors. Virtually anything that could be thought up for the treatment of disease was tried out at one time or another, and, once tried, lasted decades or even centuries before being given up. It was, in retrospect, the most frivolous and irresponsible kind of human experimentation, based on nothing but trial and error, and usually resulting in precisely that sequence. Bleeding, purging, cupping, the administration of infusions of every known plant, solutions of every known metal, every conceivable diet including total fasting, most of these based on the weirdest imagines about the cause of disease, concocted out of nothing but thin air-this was the heritage of medicine up until a little over a century ago.
–Dr. Lewis Thomas. American physician and essayist. From The Medusa and the Snail.
PRINCIPLES AND APPROACHES OF CLINICAL TRIALS
Types of Clinical Trials
Although numerous classifications have been proposed about how to categorize clinical trials, most focus on safety, efficacy, or both. All clinical trials contain at least some safety considerations, even if safety per se is not listed as an objective of the trial. While pharmacokinetics (absorption, distribution, metabolism, and excretion) may relate to safety and/or efficacy pharmacokinetics is considered as a separate type of clinical trial. Other categories of clinical trials include pharmacoeconomics, compliance, and patient-reported outcomes (i.e., quality of life).
Levels of Clinical Trials
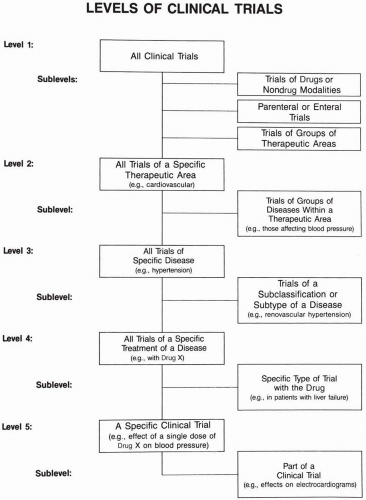
Five discrete levels of clinical trials exist and are shown in Fig. 59.1.
A number of sublevels of each of these levels are also indicated. These levels and sublevels help one understand the types
of clinical trials. Any particular clinical trial is part of multiple levels and may therefore be viewed from different perspectives. This is especially important to consider when data from different trials are compared and possibly combined.
of clinical trials. Any particular clinical trial is part of multiple levels and may therefore be viewed from different perspectives. This is especially important to consider when data from different trials are compared and possibly combined.
Caveats about This Chapter and Section
This book cannot go into great detail about the design and conduct of a single clinical trial, the interpretation of data resulting from a clinical trial, or the planning and management of multiple clinical trials. Readers who desire this information are referred to the Guide to Clinical Trials (Spilker 1991). That book focuses on Level 1 of Fig. 59.1, although numerous examples are given from all levels. Many books are written about methods, data, or drug treatment at Levels 3 and 4. There are few references of practical information on clinical methods at Level 2 (trials in a specific therapeutic area). This chapter presents general concepts and principles relating to clinical trials for individuals who seek an overview of this field.
Categories of Clinical Indications
Virtually all drugs are evaluated for activity in more than one indication (i.e., disease or medical problem). For any drug, this usually occurs sequentially for some diseases and simultaneously for others. In planning the clinical development for a drug, it is important to develop a strategy of how the drug’s clinical activity (i.e., efficacy) will be determined in terms of timing of trials and order of regulatory submissions for each indication of interest (i.e., both for different dosage forms and different countries). This strategy is part of the foundation of a drug’s development. This decision is usually made before the drug has been studied in humans, even though the strategies may be revised at later times. To assist in this effort, the possible indications to test may be divided or categorized into several groups. One such categorization is listed below, and these points may refer to a drug of the same company or of one of its competitors:
Indications already included in the labeling of a comparable drug that is already on the market
Indications that have been submitted to a regulatory agency, for either a marketed or investigational drug
Indications that are currently being evaluated for a similar drug where efficacy is established and there is a firm intention to submit a regulatory application
Indications being evaluated for an investigational drug where efficacy has not yet been well established, for which a regulatory application is planned to be submitted if safety, efficacy, and other data are acceptable
Indications that will be evaluated in pilot trials when time and resources permit
Potential indications that may be evaluated in the future
Indications for which regulatory approval will not be sought, but where clinical publications are desired
This same type of categorization could be devised for various routes of administration and/or dosage forms of a drug.
After the categorization is complete, it is possible to prioritize the order in which each specific indication will be pursued and to begin to allocate resources appropriately to pursue this effort. The overall plan may focus on choosing one indication, one route of administration, and one dosage form and not to deviate from this plan during the entire investigational period from the first human exposure until the New Drug Application is filed. This has been referred to as a laser approach (see Chapter 60). Few drugs follow such a highly focused path. Most drugs either start out with multiple indications being pursued or start out with a single indication and plans to study the drug for additional indications during the investigational period. Different visual models of these (and other) approaches are shown in Chapter 60.
Choosing the Indications to Pursue
Different groups of professionals within a pharmaceutical company often have different perspectives and opinions on this issue. Clinicians consider patient need and the therapeutic utility of a potential new drug, as well as the clarity and regulatory acceptability of the endpoints, plus ease of development. Non-clinical scientists may approach this question from a scientific value point of view. Marketing personnel emphasize commercial aspects, companion indications to products the company is already selling, and patient need. All three views may be in concert, but disagreements sometimes arise. For example, imagine in 1980 that both marketers and scientists may be enthusiastic about pursuing a new drug to treat chicken pox in children. But the pharmaceutical company’s clinicians probably realize that practicing physicians are unlikely to prescribe a new drug for most children with mild cases of chicken pox because (a) the disease is self-limiting, (b) the risk-to-benefit ratio for using the drug in children does not mandate that the disease must be treated, (c) the disease often is either not diagnosed or misdiagnosed in children, and (d) it would be necessary to prove that children treated with the drug prophylactically developed long-term immunity that was at least the same as that obtained after the disease. This is necessary because chicken pox is often a much more serious disease when it occurs initially in adulthood. Of course, Merck developed an effective vaccine that did take a significant amount of time to be accepted by most clinicians, primarily because of questions of benefit risk as discussed above.
At some companies, the indications that are pursued are primarily selected by senior research and development managers, while at other companies, medical personnel or strategic planning teams have this responsibility. It is important to develop each new drug according to a broad plan that is appropriately reviewed and accepted by all levels of the organization.
Some clinical staff may spend a significant amount of time planning unauthorized trials or evaluating indications that lie outside the approved plan. This is usually done in the hope that they will be able to eventually convince relevant managers that the indication of interest to the clinical proponent is worthwhile to develop or should be evaluated at an earlier point in the drug’s development. This practice should be strongly discouraged because it destroys the resource allocation and prioritization systems and encourages senior staff to pursue their own personal interests, usually at the expense of rapid drug development.
Another approach to choosing among indications to pursue is to develop a list of factors affecting the choice of indication, and assign each with a numerical score (or at least a qualitative one) using a Likert scale (e.g., no importance, little importance, moderate importance, or great importance). The indication(s) with the highest scores would be chosen for development. While careful thought has to be given to the weights applied to each factor, the author favors this approach when a decision on choosing among indications becomes highly complex and unclear.
How Is a Clinical Question (i.e., Objective) Posed in a Trial?
The way in which a clinical question is specifically worded in the protocol greatly influences the design of the trial that will be conducted to answer the question. A protocol is the detailed description of what, how, and why everything will be done in the clinical trial. The question(s) posed in the protocol is called the trial’s objective and is the major purpose for conducting the trial. For example, when developing a new antihypertensive drug, many different questions are asked at various stages of the drug’s development. Initial questions in Phase 1 relate to a drug’s safety, but in Phase 2, questions of efficacy become more important. To illustrate this point, two of the questions that could be the major objective of a trial and relate to efficacy are listed, along with a few comments on an appropriate trial design that would be appropriate for a trial that would seek to answer each of the two questions.
What doses of the drug cause a lowering of blood pressure by 8 to 15 mm Hg for one month? To answer this question, one would design a trial testing several doses of the drug in
patients treated for a month after they had been screened, enrolled in the trial, had other drugs removed, gone through a baseline period, and then had the drug’s dose gradually raised to a stable level. One or more control groups would likely be included. Patients in a control group would usually be given either a placebo or a different active drug.
Is the blood pressure-lowering effect of the drug the same for patients who are treated twice a day as for those who receive the same total dose divided into four individual doses per day? To answer this question, one would design a trial comparing effects of two similar groups of patients given the two different dosage regimens. It is possible that, at the end of a specific period of time, each patient would be switched to alternate treatment. If the trial was to be double-blind, then each patient would take drug four times a day, but those on the twice-a-day regimen would take the active drug twice a day and a placebo twice a day, although they would be unaware of taking a placebo. This would either require a special packaging of the drug (e.g., blister pack where each day’s dose is labeled) or the use of two different bottles of drugs. The blister pack method is easier for patients to use and would most likely enhance their compliance. A different trial design using a separate group of patients for each treatment regimen could also be used.
During Phase 3 and 4 trials, more sophisticated questions could be posed. For example, how many years of antihypertensive treatment with a specific drug are required to prolong a patient’s life by one year? Do patients who receive a specific antihypertensive drug treatment live longer than patients who do not take drug therapy? These examples illustrate that the trial design chosen depends on the question asked and that it is necessary to phrase a trial’s objectives as precisely as possible, and not merely state that the question is to evaluate efficacy of the drug in hypertensive patients.
How Many Questions May Be Asked in a Single Clinical Trial?
There is a strong temptation when designing a clinical trial to ask too many questions. Because it is possible to design a trial that asks numerous questions, many people from fields outside of medicine (e.g., science, marketing) believe that it is not only possible but is also better to conduct a single large trial to answer many questions, rather than conducting a series of trials (which may or may not be smaller in size) but only ask one or possibly two major questions in each. This desire is like the Siren luring Ulysses. It is an alluring prospect but will probably lead to serious problems and could lead to a drug’s destruction on the rocky shore. Others may see this issue as deciding whether to gamble the drug’s future on a large, risky trial that will save development time if it works, rather than being more conservative and conducting several smaller trials that will probably require more time to complete. However, it is often necessary to conduct several small trials as opposed to a single larger one, and this is usually a more sound approach to drug development. A risk taker can gamble that a go-for-broke approach will be successful and seek to only conduct a few major trials, rather than the step-wise approach usually favored by the Food and Drug Administration (FDA) and other agencies.
When a number of factors are varied in a single trial, the specific reason for a particular clinical outcome is often unclear. For example, assume that two important factors are changed in a trial of an active drug. Also assume that the new clinical results do not demonstrate that the drug worked. Then, it is usually uncertain as to which of the two factors was responsible for the failure to demonstrate drug activity. As an illustration, if an investigational drug was shown to be effective when a 500-mg dose was given four times a day, it might be important to learn if a 300-mg dose would be active and also if dosing patients twice a day would be effective. A trial that evaluated a 300-mg dose given twice a day could be designed and conducted. If the drug was inactive, however, it would not be known whether the loss of activity was because the dose was lowered from 500 to 300 mg or because the frequency of dosing was lowered from four to two times a day (or both). This simple example illustrates how critical it is to design clinical trials that do not attempt to accomplish too many major objectives in a single trial.
The example given is similar to a situation that occurs thousands of times every day when patients visit their physicians. Assume that a patient is taking a drug that is not working effectively or is causing adverse events. The physician may alter several parameters at once and hope that the patient’s situation will improve. These parameters could include adding a new drug, stopping the main drug, changing the dose of the main drug, changing the dose of a concomitant drug that may be interacting with the main drug, changing the patient’s diet or level of physical activity, and so forth. If the patient improves, it is usually unclear which specific change or combination was responsible. If the patient does not improve, it is still possible that only making a single change would have led to improvement. Further, if an unexpected outcome occurs, then it will be totally unclear as to which factor(s) are responsible. On the other hand, it is usually extremely difficult for a physician (unlike a scientist) to change only one parameter at a time. Patients are often unwilling to return each week as the physician slowly evaluates many possibilities over a period of months, although this situation does occur when an allergist seeks to identify an offending allergen. A balance between these extremes must be sought.
Posing Questions about Adverse Events
The way a trial is designed, conducted, analyzed, and interpreted relates to that question or objective. If the most appropriate question to ask is not posed, then the best data and answers are usually not obtained. As an example, the phrasing of each of the following nine questions about a drug’s adverse events would influence the trial design chosen to answer that question:
What types of adverse events does Drug X cause?
Did Drug X cause adverse event Y in Patient Z?
Does Drug X cause adverse event Y in patients with Type A disease?
What types of adverse events does Drug X cause in Patient Population A?
Does the route of administration affect the adverse events caused by Drug X?
Does the dosage form of Drug X affect the type (or number) of adverse events it causes?
Does Drug X cause more adverse events than does Drug C?
Does Drug X cause more serious adverse events than does Drug C?
Does Drug X cause more serious adverse events of Type H than does Drug C?
How Are Appropriate Clinical Trials Chosen?
A few basic approaches primarily determine the types of clinical trials conducted and designs used. These include the following approaches for new drugs:
Determine the type of labeling desired. This usually starts with identifying the specific indication(s) to be targeted. This points the project in a certain direction in terms of the type of evidence and clinical trials needed to obtain the required data. It is important for the marketing group to be aware that their advertising is directly related to the label (e.g., if a company wishes to promote a benefit of their drug it must be stated in the label).
Determine how many indications, dosage forms, and routes of administration are desired in the first group of regulatory submissions. Evaluate the total amount of resources available and the priority of the drug compared with others in the company’s portfolio to determine the amount of resources to be allocated to its development.
Determine the types and amount of data needed to get a drug approved most rapidly. This includes consideration of the patient population(s) to be studied. Then work backward to determine each of the clinical trials needed. Certain assumptions are usually made, such as the necessity in most instances to conduct two well-controlled trials for each indication desired. Major modifications to these and other assumptions need to be discussed and agreed with regulatory agencies.
Each of these three approaches allows a clinical plan to be developed that proceeds from one’s current position to the goal (i.e., regulatory approval). (Of course, not all companies have a goal of taking a product all the way through development to market approval, particularly those that intend to seek a partner to license the drug after they have achieved a certain milestone.)
Not all companies pursue this type of systematic and logical approach to drug development. A few companies have been known to initially sponsor academic-type clinical trials (e.g., mechanism of action) or to focus on conducting multiple pharmacokinetic trials at an early stage. Others approach trial designs in a random manner or follow another pattern (see Figs. 59.2 and 59.3). The number of drugs “developed” in this inappropriate manner is fortunately diminishing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree