Chapter 6 Spongiotic, psoriasiform and pustular dermatoses
Eczema – general considerations
The earliest clinical lesions are erythema and aggregates of tiny pruritic vesicles, which rupture readily, exuding clear fluid, and later become encrusted (Fig. 6.1). More chronic lesions become scaly and thickened (lichenification), resulting in lichen simplex chronicus (Fig. 6.2). Lichenification occurs if the skin is continually scratched or rubbed as, for example, in atopic dermatitis. Therefore, the clinical features of dermatitis depend upon the duration of the lesions, site(s) involved, and the amount of scratching.
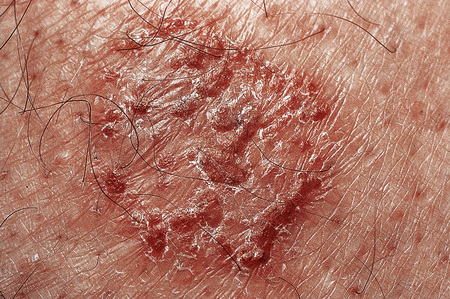
Fig. 6.1 Eczema: this is a plaque of discoid eczema. Small vesicles are present at the edge of the lesion.
By courtesy of the Institute of Dermatology, London, UK.
Eczematous dermatitis has two major etiological classifications:
Endogenous dermatitis
Atopic dermatitis
Clinical features
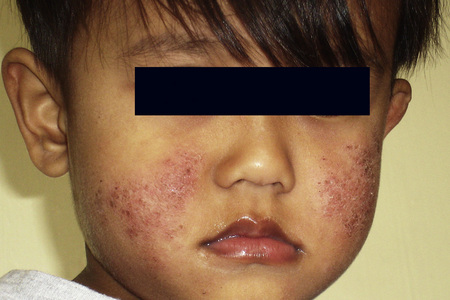
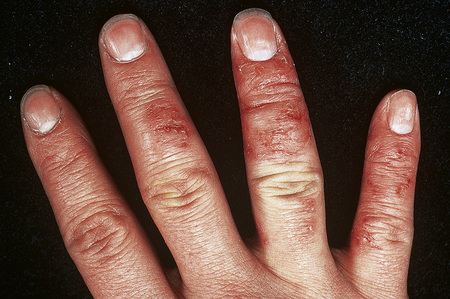
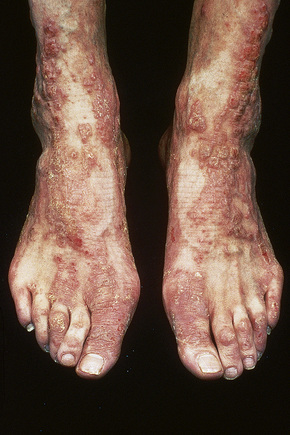
Although atopic (infantile or flexural) dermatitis may begin at any age, it usually commences from about the sixth week onwards. It is characterized by a chronic, relapsing course.1 In the infantile phase lesions are present mainly on the head, face, neck, napkin area, and extensor aspects of the limbs (Fig. 6.3). As the patient grows older and enters childhood, the eruption shifts to the flexural aspects of the limbs. Chronic atopic cheilitis may also be evident.1 Pruritus is intense and constant scratching and rubbing leads to lichenification and frequent bouts of secondary bacterial infection (Fig. 6.4).2,3 Atopic eczema is commonly associated with dry skin (xerosis). Vesiculation is uncommon. There is an increased risk of dermatophyte and viral infections.1
The disease improves during childhood and, in over 50% of cases, clears completely by the early teens. Approximately 75% of patients with atopic dermatitis have a family history of atopy and up to 50% have associated asthma or hay fever.4,5 The condition typically worsens in the winter months. It is associated with an increased incidence of contact dermatitis, particularly affecting the hand.6 Other features that may be seen include ichthyosis (50%), nipple eczema, conjunctivitis, keratoconus, bilateral anterior cataracts, sweat-associated itching, wool intolerance, perifollicular accentuation, food intolerance and white dermatographism.5 Infraorbital folds (Dennie-Morgan folds) are said to be characteristic of atopic dermatitis, particularly when double.1
Pathogenesis
Atopy is defined as a genetically determined disorder encompassing dermatitis, asthma, and hay fever. It is associated with excess immunoglobulin E (IgE) antibody formation in response to common environmental antigens. A subset of patients with ‘intrinsic atopic dermatitis’ represents perhaps 10–30% of patients with atopy; this does not appear to be due to a response to an environmental antigen.7 Atopic dermatitis is a multifactorial disease. Its pathogenesis is complex and, despite recent advances, only incompletely understood. In addition to a genetic susceptibility, the main elements responsible for the initiation and maintenance of the disease state include abnormal skin barrier function, abnormal activity of the innate and adaptive immune systems, as well as environmental factors and infectious agents.7–15
Since patients with atopic dermatitis often have a personal or family history of asthma or allergies, a genetic predisposition to the disease has long been suspected. Recent studies have demonstrated that loss-of-function mutations in the FLG gene, encoding the cornified envelope protein profilaggrin, are present in a significant subset of patients with atopic dermatitis and represent the highest risk factor for development of the disease.16 Together with involucrin and loricrin, filaggrin is a major constituent of the cornified envelope during terminal keratinocyte differentiation responsible for intact epidermal barrier function. Disruption of the skin barrier function appears to be of particular significance in the initiation and early stages of the disease. Nevertheless, 40% of patients with FLG mutations never develop atopic dermatitis and FLG mutations have been identified in only 14–56% of patients, indicating that other factors may also play an important role in the pathogenesis of the disease. In addition to the cornified envelope, epidermal barrier function is maintained also by other factors such as proteases and protease inhibitors as well as direct keratinocyte–keratinocyte interaction. Increased expression of kallikrein-related peptidases has been observed in the stratum corneum in atopic dermatitis and in one study a 4-bp insertion into the 3′ untranslated region of KLF7 leading to increased levels of this protease was identified in patients with atopic dermatitis.13,17,18 However, this finding has not been substantiated in further studies.13 Linkage has also been demonstrated to the gene SPINK5, encoding the serine protease inhibitor LEKTI. LEKTI, expressed at the granular cell layer, is an important inhibitor of the kallikrein-related peptidases KLK5 and KLK7 and is responsible for controlling desquamation. Linkage to SPINK5 is, however, significantly weaker than to profilaggrin.13 A further mechanism involved in skin barrier function is the presence of intercellular junctions, and recent data have demonstrated reduced expression of the tight junction protein claudin-1 in atopic dermatitis.15
Many lines of evidence also implicate an abnormal immune response as pivotal in the pathogenesis of atopy. It is interesting to note that atopy is cured by bone marrow transplantation in patients with Wiskott-Aldrich syndrome, an immunological disorder characterized by susceptibility to infection and thrombocytopenia, in addition to eczematous dermatitis.19 Wiskott-Aldrich syndrome shows an X-linked recessive pattern of inheritance and is characterized by depletion of nodal and circulating T lymphocytes. Contrariwise, patients without a prior history of atopy may develop atopic disease following transplantation of bone marrow from an atopic individual.20
Patients with atopic dermatitis have an abnormal immune reaction to a variety of environmental antigens leading to production of IgE antibodies and a T-cell response.9,21–23 There is evidence that certain subpopulations of T cells selectively circulate to and perform immune surveillance for the skin and lymph nodes that drain cutaneous sites.9,23 This subset of lymphocytes is characterized by a unique immunophenotype and is defined by expression of cutaneous lymphocyte antigen (CLA). In patients with atopic dermatitis, antigens such as dust mites and bacteria activate CLA T cells, resulting in the production of cytokines, which stimulate eosinophils to produce IgE, which, in turn, promotes mast cells and basophils to release cytokines and chemotactic factors in what has been termed the intermediate-phase response.8 The so-called late-phase reaction is characterized by migration of eosinophils, lymphocytes, histiocytes, and neutrophils from the circulation into the dermis and epidermis.
Factors released by the various cells present in the dermis certainly play a role in the generation of the clinical appearance and induction of pruritus, leading to scratching and rubbing. In the early phase, mechanical trauma and skin barrier disruption lead to release of proinflammatory cytokines (IL-1α, IL-1β, TNF-α, GM-CSF) which activate cellular signaling and induce expression of vascular endothelial cell adhesion molecules after receptor binding to endothelial cells.15,24,25 These steps subsequently initiate transvascular migration of inflammatory cells.8,26 Chemokines released by inflammatory cells attract a more directed cellular immune response. In particular, CCL27, CCL22, and CCL17 are increased in patients with atopic dermatitis and levels correlate with disease activity.14,25 Disease onset is related to TH2 cytokines IL-4, IL-13, and IL-31 while disease maintenance (chronic phase) is associate dwith TH1 cytokines. Other T cells, such as Treg and TH17, are also present in cutaneous lesions but their precise role is uncertain.14 The demonstration that squamous cells in patients with atopic dermatitis show increased production of GM-CSF, a cytokine thought to play a role in Langerhans/dendritic cell function, further suggests that a keratinocyte defect may be involved in the pathogenesis of atopy.27
Another area of interest has been the role of superantigens in the pathogenesis of atopy as well as other immunologically mediated cutaneous and noncutaneous disorders.28–33 Although superantigens have been implicated in the pathogenesis of psoriasis and Kawasaki’s disease, in addition to atopic dermatitis, their precise role in these and other diseases is not well understood and is controversial.29,30 Further research is necessary to clarify the role of superantigens in immunologically mediated diseases.
Superantigens are microbiological (viral, bacterial, fungal) toxins that stimulate CD4+ T cells. They bind to T-cell receptors and to the class II major histocompatibility complex (MHC), thus stimulating lymphocyte proliferation, activation and release of cytokines, as well as T-cell-mediated tissue damage. They may also stimulate B cell activation. Superantigens are powerful mediators of the immune system by virtue of their ability to stimulate a large population of T cells in a non-specific manner. Staphylococcal superantigens have, in particular, been an area of research.32 The skin of most patients with atopic dermatitis is colonized with Staphylococcus aureus. In contrast, S. aureus is found on the skin of only a minority of control subjects.33 Disease severity has been shown to correlate with the presence of toxigenic S. aureus.34 It is thought that staphylococcal superantigens SEA and SEB (staphylococcal enterotoxins A and B, respectively) activate T cells.34–37 In a study of children with atopic dermatitis, there was a correlation of disease severity and presence of SEA and SEB antibodies.35 Recently, application of SEB was shown to be associated with T-cell activation in both normal and atopic patients.38 In summary, there is mounting evidence that staphylococcal superantigens play a role in the symptomatology of atopic dermatitis. Whether superantigens play a key role in the development of disease or simply exacerbate symptoms in atopic patients requires further study.
Seborrheic dermatitis
Clinical features
Seborrheic dermatitis is a common dermatosis which affects up to 1–3% of the population.39–41 There is a male predominance. It presents in infants, with a second peak affecting adults.42 There is often a family history of the disease. It particularly affects those areas where sebaceous glands are most numerous, i.e., the scalp, forehead, eyebrows, eyelids, ears, cheeks, presternal and interscapular areas (Figs 6.5, 6.6).43 Occasionally, the flexural regions are affected (intertrigo). Often the lesions of seborrheic dermatitis are sharply marginated, dull red or yellowish, and covered by a greasy scale.43 They are therefore easily confused with psoriasis.
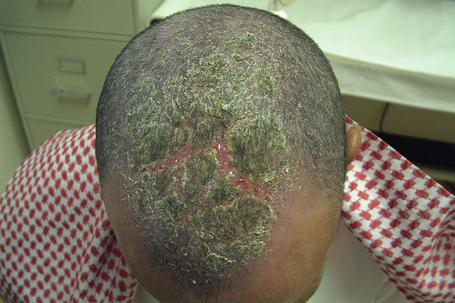
Fig. 6.5 Seborrheic dermatitis: there is diffuse erythema and scaling of the scalp.
By courtesy of B. Al Mahmoud, MD, Doha, Qatar.
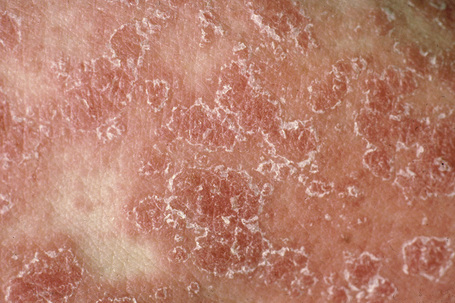
Fig. 6.6 Seborrheic dermatitis: note the marked scaling.
By courtesy of the Institute of Dermatology, London, UK.
Dandruff and cradle cap are also sometimes included within the spectrum of seborrheic dermatitis.
Seborrheic dermatitis is one of the most common dermatoses seen in patients with acquired immunodeficiency syndrome (AIDS). Seborrheic dermatitis has also been associated with stress and neurological disorders including Parkinson’s disease, syringomyelia, and trigeminal nerve injury.44
Pathogenesis
The precise pathogenesis of this condition is unknown. Surprisingly, and in spite of the distribution (and the name) of the disease, sebaceous gland activity and sebum composition appear to be normal.44
Seborrheic dermatitis is associated with heavy colonization of the skin by the lipophilic yeast Malassezia furfur (Pityrosporum ovale) while more recent data have identified a predominance of Malassezia restricta and Malassezia globosa.41,45–50 Although many workers in the field believe this to be of etiological importance, an almost equal number are unconvinced. The body of evidence favoring a significant relationship relates to the successful treatment of seborrheic dermatitis with antifungal therapy.39,40,51 Whether this implies a causal relationship or merely an exacerbating factor is, however, uncertain.
Discoid dermatitis (nummular eczema)
Clinical features
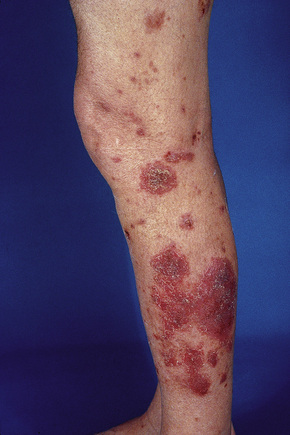
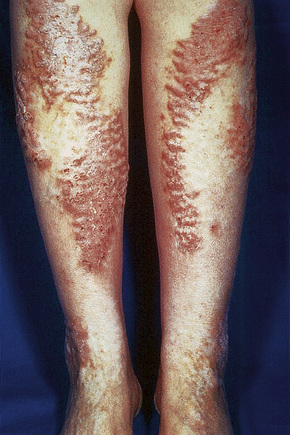
The presence of single or multiple pruritic, coin-shaped, erythematous plaques with vesiculation, particularly involving the lower legs, forearms, and backs of hands (Figs 6.7–6.9) characterize this chronic form of dermatitis.52 The absence of a raised border clinically distinguishes it from ringworm.52 There are two peak ages of onset: it affects young women (15–30 years of age) and middle-aged adults of both sexes. The disease tends to chronicity.
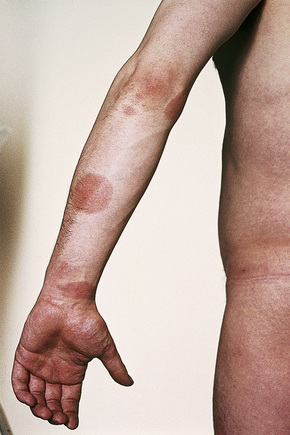
Fig. 6.7 Discoid eczema: circumscribed, erythematous lesions on the forearm, a characteristic site.
By courtesy of the Institute of Dermatology, London, UK.
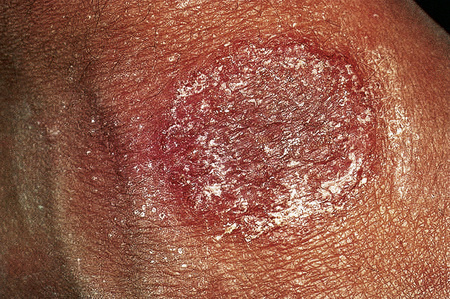
Fig. 6.8 Discoid eczema: the lesion is sharply defined and there is a pronounced scale.
By courtesy of the Institute of Dermatology, London, UK.
Pathogenesis
The pathogenesis is poorly understood. A participatory role for organisms in the pathogenesis has been suggested but not been widely accepted.53 Discoid dermatitis may follow irritants such as soap, acids or alkalis (Fig. 6.10).52 Sometimes it may be a manifestation of atopy and, occasionally, it develops as a consequence of nickel, chromate or cobalt allergy.54,55 Generalized disease has also been documented in the setting of interferon alpha-2b plus ribavirin treatment for hepatitis C infection.56
Hand eczema (dyshidrotic eczema, palmoplantar eczema, pompholyx)
Clinical features
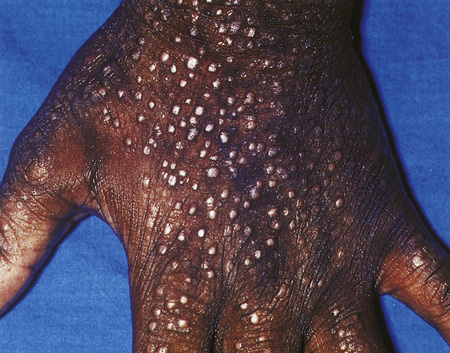
Hand eczema is characterized by a recurrent pruritic vesicular eruption of the palms, soles or digits. Because of the increased thickness of the keratin layer at these sites, the vesicles appear as small pale papules before rupturing (Figs 6.11, 6.12). Occasionally, frank bullae can form. With the passage of time, the affected parts may show scaling and cracking. The nails sometimes become dystrophic, with discoloration and transverse ridging.57 In the majority of cases the cause is unknown, although heat or psychological stress may precipitate an attack.57 In some patients there is a personal or family history of atopy or coexisting tinea pedis. Rubber, latex, chromium, cobalt or nickel sensitivity may be the trigger.58–62 The condition can be exacerbated by heat and, rarely, it is photoinduced.63
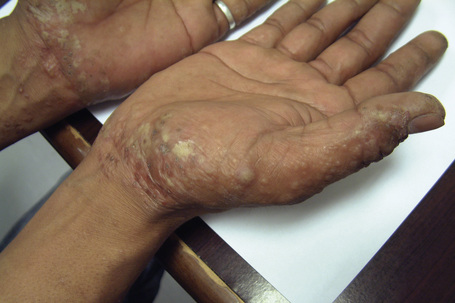
Fig. 6.11 Hand eczema: tense yellow vesicles are present.
By courtesy of B. Al Mahmoud, MD, Doha, Qatar.
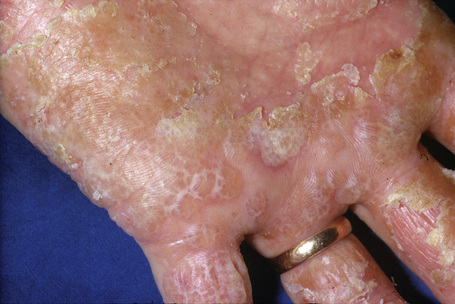
Fig. 6.12 Hand eczema: more chronic example showing marked scaling.
From the collection of the late N.P. Smith, MD, the Institute of Dermatology, London, UK.
Pompholyx is often associated with hyperhidrosis.58 Females are affected slightly more often than males and patients are predominantly in the second to fifth decades.59 A familial autosomal dominant form has been reported where the candidate gene has been mapped to chromosome 18q22.1-18q22.3.64
Autosensitization (Id) reaction
Clinical features
On occasion, patients will develop generalized spongiotic dermatitis in response to a dermatosis or infection at a distant site. The eczematous dermatitis resolves if the underlying infection or specific dermatosis is successfully treated. This phenomenon has also been designated an autoeczematization or Id reaction. The lesions that characterize the Id reaction may be a localized pompholyx-like eczematous dermatitis of the hands and feet or scattered papules on the trunk and limbs.65–69 Disorders that may be associated with the Id reaction include fungal infection (e.g., dermatophyte infection), scabies infestation, molluscum contagiosum, tick bite, pediculosis capitis, and bacterial and mycobacterial infections.65–69 A generalized nummular dermatitis has been reported in association with localized dental infection.70
Exogenous dermatitis
Contact dermatitis
Allergic contact dermatitis
Allergic contact dermatitis is an idiosyncratic cell-mediated immunological reaction to an environmental allergen, which may be present in very low concentration. Common examples seen in clinical practice include sensitivity to nickel (found in items such as jewelry, buttons, watches, and suspenders), constituents of synthetic rubber (e.g., thiuram in rubber gloves), primula, poison ivy, topical medicaments (e.g., neomycin, antihistamines, local anesthetics), and chromates found in cement and leather (Figs 6.13–6.15).72–82
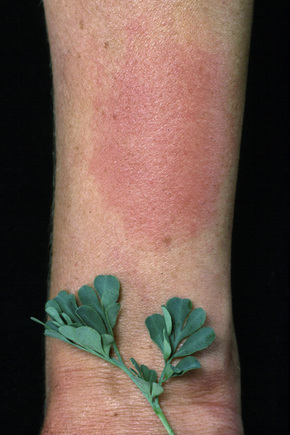
Fig. 6.15 Contact dermatitis: a severe reaction to poison ivy.
From the collection of the late N.P. Smith, MD, the Institute of Dermatology, London, UK.
Dinitrochlorobenzene (DNCB) is a potent contact sensitizer and this is used as a test of cell-mediated immunity.83,84
A growing understanding of allergic contact dermatitis has emerged over the last decade with the preponderance of evidence pointing to a T-cell-mediated hypersensitivity reaction.3,85–88 It is thought that antigens causing allergic contact dermatitis are often unstable (haptens) and need to bind to epidermal host proteins.3,85 These hapten–protein interactions are formed via covalent binding of electrophilic residues of the chemical with amino acids, especially cysteine.89–91 In contrast, metal ions, such as nickel, are thought to form noncovalent protein–metal chelate complexes.89 In the sensitization/initiation phase of the disease, hapten-specific T cells are generated in lymph nodes after the initial contact of the skin with a potent hapten.91 Langerhans cells in skin and dendritic cells in lymph nodes process antigen and stimulate appropriate naive CLA T cells. CLA-positive T cells are a subset of T cells that express a skin-selective homing receptor and perform immune surveillance for the skin and lymph nodes that drain cutaneous sites.92 CLA-positive T cells proliferate when stimulated by the appropriate antigen or antigen–protein complex. The number of CLA-positive memory T cells increases with repeated exposures to its antigen.3,85 When the patient is exposed to the antigen, the elicitation phase, the CLA-positive T cells are activated and release cytokines which lead to the immune reaction responsible for the clinical and histological features associated with allergic contact dermatitis.87 CD8+ T cells appear to be the main effector cell in the elicitation phase.89 Keratinocytes are also thought to play a role through the release of cytokines after hapten exposure and binding.88
Occasionally, exposure to strong haptens may result in the development of allergic contact dermatitis in previously unsensitized individuals (primary allergic contact dermatitis).89
Ingested or inhaled allergens in a person who has been previously sensitized by cutaneous absorption may result in a clinical picture similar to allergic contact dermatitis (e.g., ingested nickel, chromium or cobalt may result in the appearances of hand eczema).93 Much less commonly, systemic allergic contact dermatitis may be histologically associated with an erythema multiforme-like eruption, vasculitis or urticarial morphology.93 This is thought to result from systemic exposure of a hapten via hematogenous transport to the skin.94 It may occur with or without prior sensitization, and a large number of drugs have been implicated in the pathogenesis.94
Irritant contact dermatitis
Irritant contact dermatitis, which is much more common than allergic contact dermatitis, follows exposure to physical or chemical substances capable of direct damage to the skin. Mechanisms of damage are variable and include keratin denaturation, removal of surface lipids and water-holding substances, damage to cell membranes, and/or direct cytotoxic effects.95 Acute irritant dermatitis usually results from a relatively short single exposure to a potent irritant, such as strong acid or alkali, whereas chronic cumulative insult or ‘wear and tear’ dermatitis is due to more prolonged contact with one or more weaker irritants, for example, soap and water, detergents or industrial oil and plants (Fig. 6.16).96–100
Similar to atopic dermatitis, loss-of-function mutation in the FLG gene, encoding filaggrin, may confer an increased susceptibility to chronic irritant contact dermatitis evoking an underlying skin barrier defect in the pathogenesis of the disease.101
Infective dermatitis
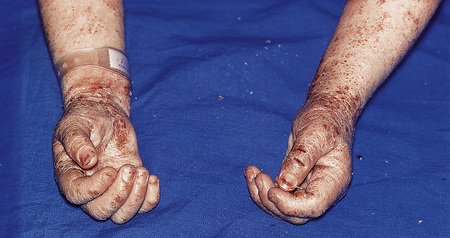
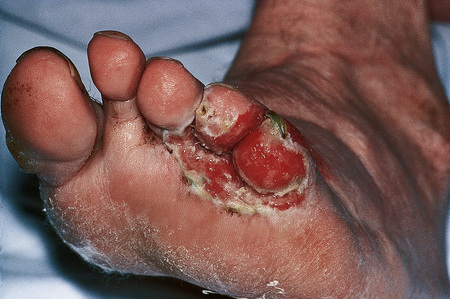
Infective dermatitis is a severe chronic and recurrent eczematous dermatitis showing pronounced exudation and crusting and presenting in children with human T-cell lymphotropic virus type 1 (HTLV-1) infection.102–105 Adult onset is exceptional.106,107 The disease has a predilection for the scalp, flexures, the ears and feet, and sometimes around wounds and ulcers (Fig. 6.17), and is frequently associated with Staphylococcus aureus and beta-hemolytic Streptococcus infection of the skin and nasal vestibule. It occurs in regions where HTLV-1 is endemic. It has frequently been reported in Jamaica, while presentation in Japan appears relatively rare.108 Development of the disease may be associated with a defective immune system and may be a risk factor for the development of other HTLV-1-related diseases such as adult T-cell leukemia and tropical spastic paraparesis.109,110
Asteatotic dermatitis
Commonly seen in the elderly, particularly in winter and in those with minor degrees of ichthyosis, asteatotic dermatitis (eczema craquelé) may be precipitated by excessive washing, exposure to detergents, cold winds or low humidity, all of which tend to dry the skin.111 The affected regions are inflamed and criss-crossed by scaly lines and superficial fissures (Fig. 6.18). Asteatotic dermatitis may be associated with internal malignancy, including lymphoproliferative disorders and solid tumors.112–115
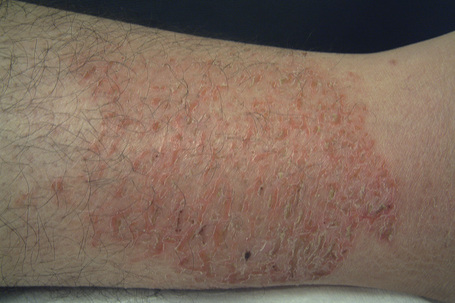
Fig. 6.18 Asteatotic dermatitis: these typical appearances are the result of scaling and fissuring.
By courtesy of B. Al Mahmoud, MD, Doha, Qatar.
Pathogenesis and histological features
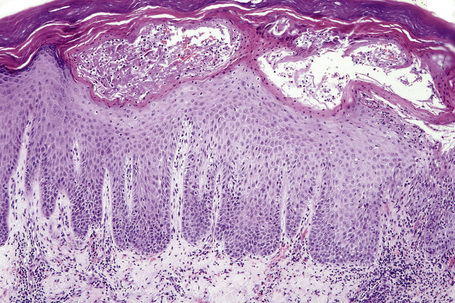
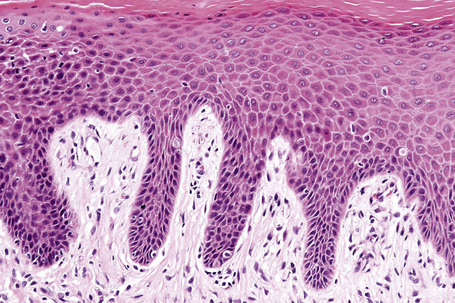
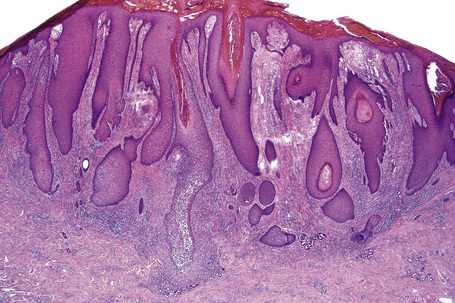
The histopathological features of spongiotic dermatitis include both dermal and epidermal changes. Their relative proportions vary to some extent with the subtype, but perhaps more importantly, with the stage of evolution of the disease. It is essential not to consider the changes of spongiotic dermatitis as static: different features are seen at different stages.116–118 Attempting to distinguish the various clinical subtypes based on histological features alone is generally futile. Instead, once the disorder has been recognized as spongiotic in nature, clinical examination is a much more satisfactory method of determining the particular variant.
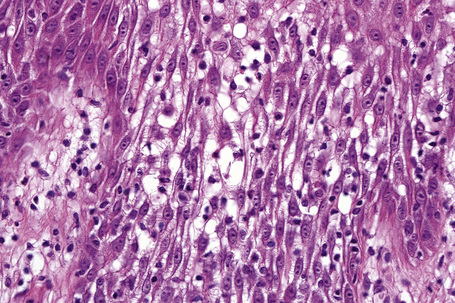
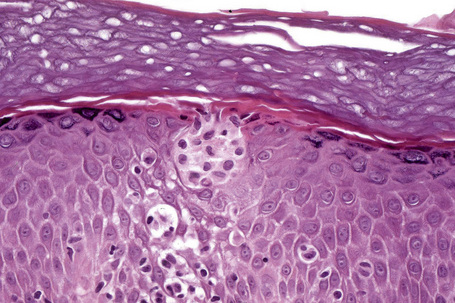
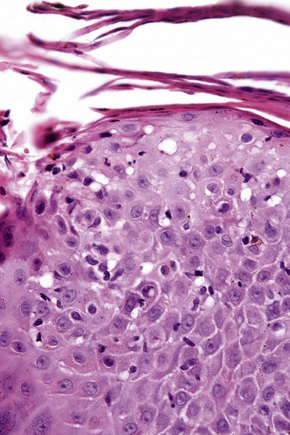
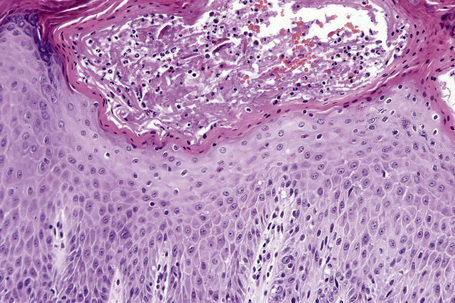
The histological hallmark of spongiotic dermatitis is the presence of intercellular edema or spongiosis (L., Gr. spongia, sponge). Slight degrees of intracellular edema may also be evident but may easily be overlooked. In the early stages of development, spongiosis results in widening of the intercellular spaces, rendering the intercellular bridges conspicuous (Fig. 6.19). Further accumulation of fluid leads to the eventual development of an intraepidermal vesicle. A common finding in association with the intercellular edema is lymphocytic infiltration of the epidermis (exocytosis). In severe contact irritant dermatitis, the epidermis may be infiltrated by large numbers of neutrophil polymorphs in association with necrotic keratinocytes.119 In addition, such reactions may be accompanied by dermoepidermal separation resulting in a vesicle or blister. The lesions very often become traumatized and may show marked crusting.
The dermis is often congested and edema is usually marked in active lesions. The vessels of the superficial vascular plexus are surrounded by a mixed inflammatory cell infiltrate composed of lymphocytes, histiocytes, and occasional eosinophils or neutrophils. The degree and composition of dermal inflammation is highly variable. Eosinophils may be numerous in allergic contact dermatitis.119
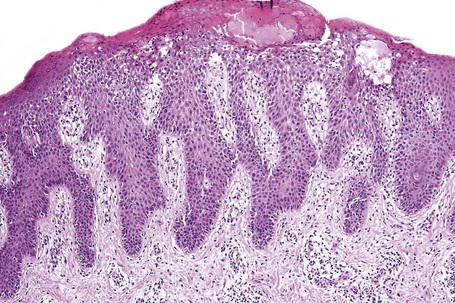
Systemic contact dermatitis may be associated with the features of vasculitis or erythema multiforme.120 As with other forms of spongiotic dermatitis the histological appearances can be divided into acute, subacute, and chronic forms. Spongiosis is more conspicuous in the acute phase although it is never marked. In contrast, the epidermal hyperplasia becomes more conspicuous and psoriasiform towards the chronic end of the spectrum.
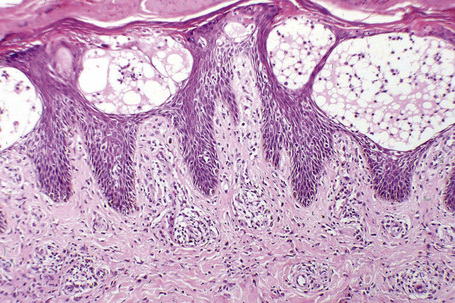
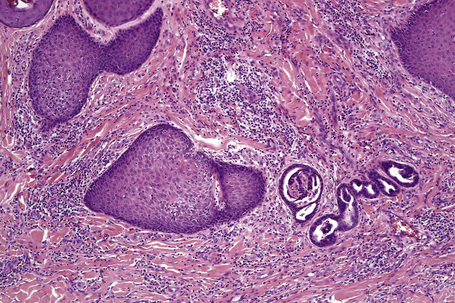
The features of seborrheic dermatitis are often non-specific and subtle. It is characterized by hyperkeratosis and parakeratosis, the latter particularly related to hair follicles and typically associated with neutrophil exocytosis (Figs 6.25, 6.26). Yeasts may sometimes be found in the stratum corneum particularly if PAS stained sections are examined. Epidermal acanthosis with thickened rete ridges is present and often marked in chronic lesions. It is, however, somewhat irregular in contrast to the uniform hyperplasia characteristic of psoriasis. Variable spongiosis with lymphocyte exocytosis is common. The dermis may be edematous and mild vascular dilatation is usually seen. A mixed inflammatory cell infiltrate consisting of lymphocytes, histiocytes, and small numbers of eosinophils surrounds the superficial vascular plexus.
Differential diagnosis
Although spongiosis is a characteristic feature of spongiotic dermatitis, it is also encountered in many other inflammatory dermatoses (Table 6.1), particularly superficial dermatophytoses. A diagnosis of spongiotic dermatitis should never be made until a stain for fungus (e.g., PAS reaction) has been performed to exclude this possibility. This is especially important since the common treatment of spongiotic dermatitides – topical corticosteroids – would exacerbate a fungal infection (tinea incognito) (Figs 6.27–6.29).
Table 6.1 Conditions featuring spongiosis
Lichen simplex chronicus
Clinical features
The term lichen simplex chronicus (circumscribed neurodermatitis) refers to the development of localized areas of thickened scaly skin complicating prolonged and severe scratching in a patient with no underlying dermatological condition (Fig. 6.30).1 Lichenification is an identical process in which an underlying intensely pruritic dermatosis such as atopic eczema is present.2 Dermatophyte infections, stasis dermatitis, and chronic allergic contact dermatitis may also predispose to lichenification. Picker’s nodules and nodular prurigo are related conditions (see below).3
Patients present with profound pruritus and localized scaly plaques with accentuated skin markings described as resembling tree bark. There is a predilection for females, and young to middle-aged adults are predominantly affected. Accessible skin is particularly affected and the nape and sides of the neck, the thighs, the lower legs and ankles, vulva, and scrotum are sites generally involved.2
Pebbly lichenification refers to a distinct variant in which lichenoid papules follow intense scratching in patients with inflammatory dermatoses such as atopic eczema.2
Histological features and pathogenesis
Although the etiology and pathogenesis of lichen simplex chronicus remains elusive, psychological factors may play an important role.4,5 Recent data further suggest that an underlying neuropathy may be of importance in at least a subset of patients.6
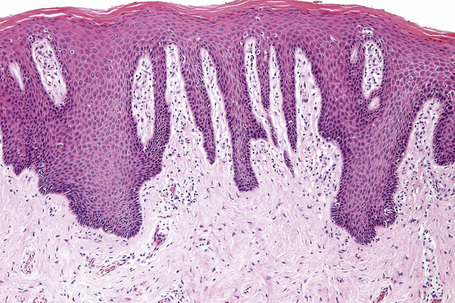
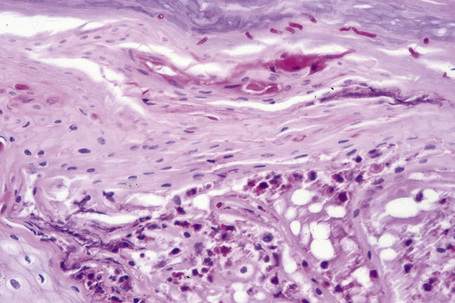
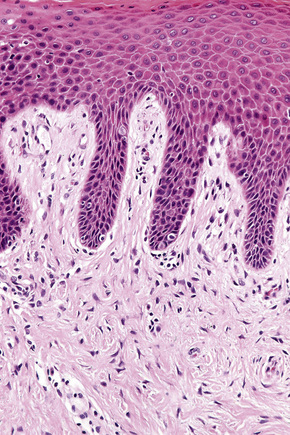
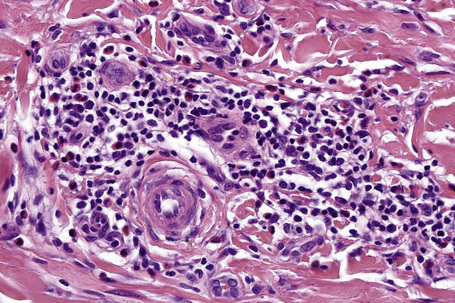
Histologically, lichen simplex chronicus is characterized by marked hyperkeratosis, sometimes with small foci of parakeratosis, and a usually prominent granular cell layer (Fig. 6.31).7 The epidermal ridges are elongated and irregularly thickened. Mild spongiosis is variably present depending upon the cause. A perivascular and sometimes interstitial inflammatory cell infiltrate consisting of lymphocytes, histiocytes, and small numbers of eosinophils is present in the superficial dermis. Enlarged, angulated myofibroblasts are sometimes evident and, as in many other chronic skin conditions, scattered small, multinucleated cells, so-called Montgomery giant cells, are identified. Papillary dermal fibrosis is a characteristic feature and in some cases nerve hyperplasia is seen (Fig. 6.32).3 In our experience, however, the latter feature is distinctively uncommon.
Nodular prurigo (prurigo nodularis) and prurigo nodule (picker’s nodule)
Clinical features
Nodular prurigo is characterized by the development of chronic, intensely pruritic, lichenified, and excoriated nodules.1,2 It occurs over a wide age range, from 5 to 75 years, with a mean of 40 years. Rarely, children are affected.3 Disease duration ranges from 6 months to 33 years, with a mean of 9 years. Nodular prurigo occurs equally in men and women. It shows significant overlap with lichen simplex chronicus, although this is not uniformly accepted.1,2
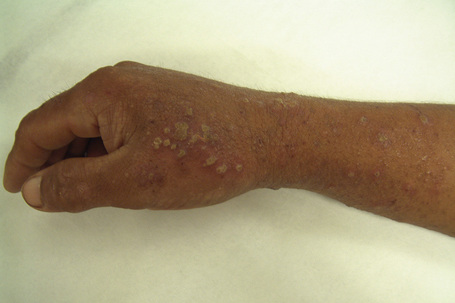
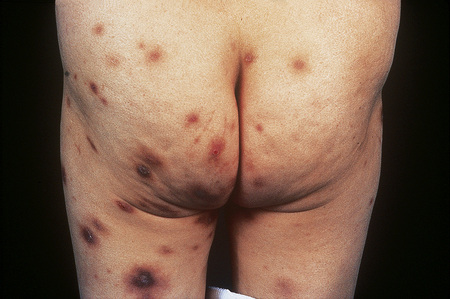
Individual lesions are often described as globular with a warty and excoriated surface and may measure up to 2 cm in diameter (Fig. 6.33).2 They are often grouped, symmetrical, and occur predominantly on extensor aspects of the (distal) limbs (Figs 6.34, 6.35).1 The trunk may also be affected.2 Occasional disseminated cases have been described.2 The palms and soles are typically uninvolved.2 The intervening skin usually appears normal. Similar-looking lesions are sometimes seen in patients with eczema (see below). The majority of patients with nodular prurigo are perfectly well and investigations are unhelpful; however, occasionally nodular prurigo is found in patients with gluten enteropathy.4 Psychosocial disorders have been reported in a high proportion of patients.5 In some cases the eruption occurs after an insect bite, but subsequent lesions develop spontaneously.5
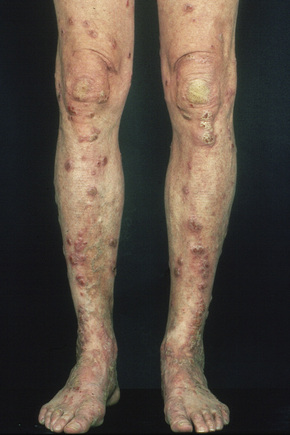
Fig. 6.33 Nodular prurigo: typical globular nodules; the intervening skin appears normal.
From the collection of the late N.P. Smith, MD, the Institute of Dermatology, London, UK.
The pruritus is episodic and may be precipitated or aggravated by heat and anxiety.5 Significant laboratory abnormalities may include anemia, eosinophilia, and raised serum IgE levels.5
Nodular prurigo (eczema) is defined as lesions of nodular prurigo arising on a background of overt eczema.5 While this distinction is of academic interest it has no clinical or prognostic importance.
On occasion, nodular prurigo is accompanied by the features of bullous pemphigoid (pemphigoid nodularis).6
Pathogenesis and histological features
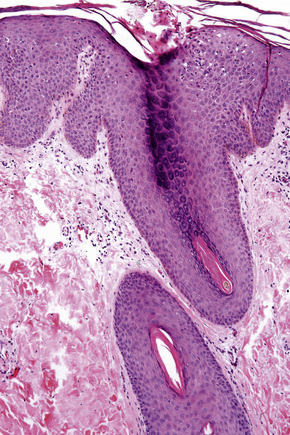
Classical nodular prurigo, which is focal and characterized by hyperplasia, has recently been related particularly to follicular epithelium.2,7 In the epidermis this manifests as orthohyperkeratosis, hypergranulosis and acanthosis, sometimes to the degree of pseudoepitheliomatous hyperplasia (Figs 6.36, 6.37).8 Superficial mild spongiosis and focal parakeratosis is occasionally present and the features may resemble chronic eczema.5 Subepidermal fibrin deposition is sometimes a feature.9
In the dermis there is vascular hyperplasia, with dilated vessels in both the papillary and reticular dermis. New vessel formation is apparent and there is a surrounding perivascular mild inflammatory cell infiltrate, consisting mainly of lymphocytes and some histiocytes, plasma cells, occasional eosinophils and scattered, superficial, small multinucleated cells (Montgomery giant cells) (Fig. 6.38).8 Mast cells are present in normal numbers.1 The infiltrate has been described as having an inverted triangular configuration extending from the superficial dermis.2 This has not been the present author’s experience. Occasionally, the dermal features include lymphoid follicles with germinal center formation, thereby resembling a persistent insect bite reaction.5 An additional finding is the presence of fibrosis of the papillary dermis.8
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree