Splenic Inflammatory Pseudotumor
Roberto N. Miranda, MD
Key Facts
Terminology
Splenic inflammatory pseudotumor (IPT)
Clinical Issues
Age: 19-87 years (median: 53 years)
Rare in children
Slight female predominance
Excision is curative
Macroscopic Features
Well-circumscribed mass
Median: 10 cm (range: 1.5-22 cm)
Microscopic Pathology
Cellular spindle cells of short fascicles with bland nuclear features
Mixed infiltrate of plasma cells, lymphocytes, and histiocytes
Ancillary Tests
Spindle cells
Vimentin(+) and CD68([+], focal)
˜ 70% of cases (+) for smooth muscle actin (focal)
CD8(-), CD21(-), CD23(-), CD30(-), desmin(-)
Lymphocytes and plasma cells
Polytypic
Molecular genetic studies
No evidence of monoclonal Ig or T-cell receptor gene rearrangements
Top Differential Diagnoses
Inflammatory pseudotumor-like follicular dendritic cell tumor
Inflammatory myofibroblastic tumor
Follicular dendritic cell sarcoma
TERMINOLOGY
Abbreviations
Splenic inflammatory pseudotumor (IPT)
Definitions
Reactive lesion of spleen composed of inflammatory cells and spindled cells with or without sclerosis
Etiology of splenic IPT is unknown
Classification is controversial since other entities have been classified as IPT; in particular
Inflammatory pseudotumor-like follicular dendritic cell tumor (IPT-FDCT)
True neoplasm that involves mainly liver and spleen
ALK(+) inflammatory myofibroblastic tumor (IMT)
Most often involves soft tissues of children and young adults
IPT-FDCT and ALK(+) IMT are now excluded from the category of IPT
ETIOLOGY/PATHOGENESIS
Infectious Agents
Etiology of splenic IPT is unknown
Most likely a number of causes may ultimately result in splenic IPT
Infectious causes are likely
Variable association reported with Streptococcus, Legionella, and Epstein-Barr virus
Vascular events may be involved
Autoimmunity has been hypothesized to play a role
Regardless of initiating event, exuberant tissue repair is probably involved in pathogenesis
Splenic IPT appears to be pathobiologically similar to IPT of lymph nodes
CLINICAL ISSUES
Epidemiology
Incidence
Uncommon; ˜ 3% of splenic masses
Rare when compared with IPT at other sites of body
Age
Range: 19-87 years; median: 53 years
Rare in children
Gender
Slight female predominance; M:F ratio = 1:1.3
Site
Typically involves spleen as single lesion
Rare cases can be multicentric
Presentation
Affected patients are immunocompetent
Fever and weight loss in about half of patients
Asymptomatic in 50% of cases
Epigastric or left flank pain, usually associated with larger lesions
Splenomegaly may be noted in some cases
Occasional IPT are associated with malignancies, e.g., colon or renal cell carcinoma
Laboratory Tests
Usually unremarkable
Occasionally patients have mild leukocytosis, (< 15 x 109/L), anemia, and hypergammaglobulinemia
Treatment
Due to rarity and nonspecific CT or MR imaging, these lesions are not diagnosed preoperatively
Diagnosis first established after splenectomy
Splenectomy is effective treatment
Symptoms and laboratory abnormalities disappear after splenectomy
Prognosis
Excellent prognosis; no deaths attributable to splenic IPT
Tumors are cured by splenectomy, and there are no reported recurrences of similar lesions elsewhere
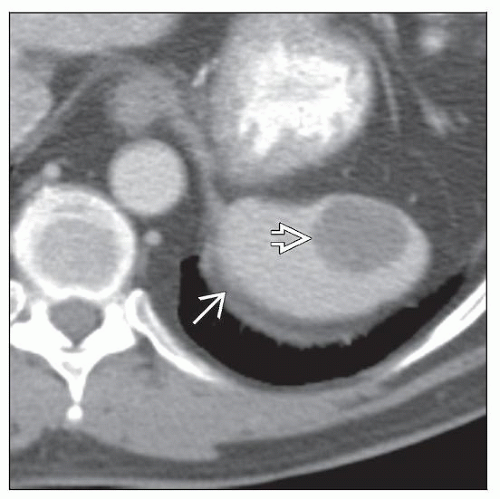
IMAGE FINDINGS
CT Findings
Discrete, single splenic mass
Associated with splenomegaly when tumors are larger
Lymphadenopathy is unusual
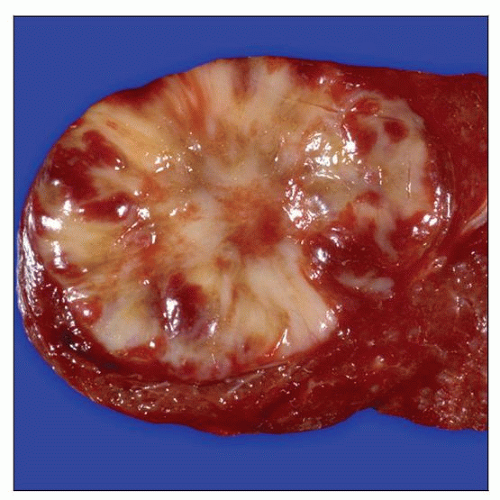
MACROSCOPIC FEATURES
General Features
Spleen weight
Mean: 331 g (range: 140-1,030 g)
Splenomegaly > 250 g in 50% of cases
Well-circumscribed, nonencapsulated single mass
Cut surface is white-tan, gray, or yellow; soft to firm lesions
Rarely multinodular
Mean size: 10 cm (range: 1-22 cm)
MICROSCOPIC PATHOLOGY
Histologic Features
Lesions are usually well circumscribed
Lesions may be partially encapsulated
Islands of white or red pulp may be trapped at periphery of lesion
3 growth patterns are recognized; may occur simultaneously
Cellular spindle cell, composed of short fascicles
Most common
Can be focally storiform; rare mitoses identified
Bland spindle cells with oval vesicular nuclei and small nucleoli
Hypocellular fibrous pattern, similar to scar tissue
Myxoid and vascularized, similar to granulation tissue
Abundant mixed inflammatory infiltrate of plasma cells, lymphocytes, and histiocytes
Variable proportions of inflammatory cells in different areas of same lesions
Marked variability from case to case
Lymphocytes are usually small with occasional immunoblasts
Mature plasma cells, with occasional Russell bodies
Other features
Focal, central necrosis usually associated with neutrophilic infiltrate
Histiocytes and eosinophils are less frequent
Hemorrhage and hemosiderin deposition
Compressed and congested splenic parenchyma around tumor; otherwise unremarkable spleen
ANCILLARY TESTS
Immunohistochemistry
Spindle cells
Vimentin(+) and CD68([+], focal)
˜ 70% of cases are positive for smooth muscle actin (focal) and desmin(-)
Smooth muscle actin(+) cells are considered myofibroblasts
Occasionally positive for S100 protein (focal) and Factor XIII
CD8(-), CD21(-), CD23(-), and CD30(-)
HMB-45(-), ALK-1(-), HHV8(-), and cytokeratin(-)
Epstein-Barr virus(+) in small subset of cases
Infected cells are spindle cells, some of which can focally express smooth muscle actin
Lymphocytes and plasma cells
Mixture of T and B cells; usually with predominance of CD3(+) cells
B cells and plasma cells are polytypic
Cytogenetics
Normal karyotype
Molecular Genetics
No evidence of monoclonal immunoglobulin (Ig) or T-cell receptor gene rearrangements
No known oncogene abnormalities
DIFFERENTIAL DIAGNOSIS
Inflammatory Pseudotumor-like Follicular Dendritic Cell Tumor (IPT-FDCT)
Female predominance
Considered variant of follicular dendritic cell sarcoma
More aggressive clinically, contrary to splenic IPT
Recurrences are common
Recurrent tumors show pleomorphic large cells usually not detected in primary tumors
Immunohistochemistry helpful as FDC can be
CD21(+), CD23(+), Factor XIII(+)
Commonly EBV(+)
Monoclonal EBV when assessing EBV DNA terminal repeat regions
IPT-FDCT of spleen is pathobiologically similar to liver IPT
Inflammatory Myofibroblastic Tumor (IMT)
Affects soft tissues of children and young adults
Ill-defined mass grossly
Myofibroblasts positive for smooth muscle actin (100%) and cytokeratin (15-30%)
Negative for follicular dendritic cell markers and H-caldesmon
Scattered large atypical cells, sometimes ganglion-like cells with prominent nucleoli
Harbors balanced translocations involving anaplastic lymphoma kinase (ALK) gene at 2p23
Anaplastic lymphoma kinase (ALK) is expressed in ˜ 50% of cases
ALK is not expressed in splenic IPT
Has locally aggressive clinical behavior with recurrences
Rare reports of IMT in spleen, but those reported have been ALK(-)
Follicular Dendritic Cell Sarcoma (FDCS)
Formerly designated as follicular dendritic cell tumor
Affects primarily lymph nodes but can involve spleen and other sites
Intraabdominal cases are often clinically aggressive
More aggressive than IPT-FDCT, with recurrences and distal metastasis
No gender predilection, except in splenic or hepatic forms where there is female predominance
FDCS can show range of histologic features
Composed of spindled or epithelioid cells
Bland or pleomorphic cytologic features
May display scattered inflammatory cells
Immunohistochemistry helpful as FDCS can be
CD21(+), CD23(+), CD35(+), CNA.42(+)
Clusterin(+), fascin(+), and EGFR(+)
Rare or no association with EBV
Inflammatory Pseudotumor (IPT) Involving Other Sites
Usually present with systemic findings; sometimes asymptomatic
IPT has been diagnosed in various anatomic sites
Respiratory tract, lungs, orbit, spinal meninges, digestive tract, heart, and lymph nodes
Encompasses lesions where myofibroblasts are detected but are not main component
Variable mix of small and activated lymphocytes
Polytypic plasma cells, histiocytes, and sclerosis
Fibrotic process in lymph node extends along capsule or trabeculae and then throughout parenchyma
EBV small encoded RNA (EBER)(+) in 20% of nodal IPT; in scattered lymphocytes
Spindle cells are negative for EBER
Sclerosing Angiomatoid Nodular Transformation (SANT)
Involves splenic red pulp
Recently described entity with overlapping features with splenic IPT
Some researchers consider that SANT is subset or end stage of splenic IPT
Single mass composed of multiple small nodules
Nodules display dense network of capillaries as well as remnants of sinuses
Endothelial cells positive for CD34 and CD31; usually negative for CD8
EBER(-); EBV latent membrane protein type 1(-)
Negative for follicular dendritic cell markers CD21, CD35, and CNA.42
Collagenous fibrosis with scattered spindle cells may occur around and in center of lesion
Spindle cells around nodules react as myofibroblasts and are smooth muscle actin(+)
Angiomatoid nodules occasionally show dense inflammatory infiltration
Polytypic plasma cells, small lymphocytes, and histiocytes are not unusual
Hyalinization of arterial walls and organizing thrombosis in veins
Splenic Hamartoma
No gender predilection
Usually found incidentally after splenectomy for other medical or surgical conditions
Sometimes found at autopsy
Involves splenic red pulp
Considered to be malformation
Usually single lesion, less commonly presents as multiple lesions
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree