Solid-state properties
Graham Buckton
Chapter contents
Key points
Solid state
The three states of matter are solid, liquid and gas (or vapour). In a sealed container, vapours will diffuse to occupy the total space, liquids will flow to fill part of the container completely, whereas solids will retain their original shape unless a compressive force is applied to them. From this simple consideration it becomes clear that solids are unique. Importantly their physical form (the packing of the molecules and the size and shape of the particles) can have an influence on the way the material will behave. At normal room temperature and pressure, the majority of drugs and excipients exist as solids, thus the study of solid-state properties is of enormous pharmaceutical importance.
Solid particles are made up of molecules that are held in close proximity to each other by intermolecular forces. The strength of interaction between two molecules is due to the individual atoms within the molecular structure. For example, hydrogen bonds occur due to an electrostatic attraction involving one hydrogen atom and one electronegative atom, such as oxygen. For molecules which cannot hydrogen bond, attraction is due to van der Waals forces. The term van der Waals forces is generally taken to include dipole-dipole (Keesom), dipole-induced dipole (Debye) and induced dipole-induced dipole (London) forces. In this context a dipole is where the molecule has a small imbalance of charge from one end to the other, making it behave like a small bar magnet. When the molecules pack together to form a solid, these dipoles align and give attraction between the positive pole of one and the negative pole on the next. Induced dipoles are where the free molecule does not have an imbalance of charge, but an imbalance is caused by bringing a second molecule into close proximity with the first.
Crystallization
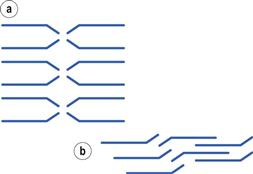
Materials in the solid state can be crystalline or amorphous (or a combination of both). Crystalline materials are those in which the molecules are packed in a defined order, and this same order repeats over and over again throughout the particle. In Figure 8.1a, an ordered packing of a molecule is shown; here the shape of the molecule is shown as a ‘hockey stick’ style image, which is representing a planar structure with a functional group pointing up at the end. This is not a real molecule – it has been drawn to provide an easy representation of a possible crystal packing arrangement. A characteristic property of a crystal is that it has a melting point. The melting point is the temperature at which the crystal lattice breaks down, due to the molecules having gained sufficient energy from the heating process to overcome the attractive forces that hold the crystal together. It follows that crystals with weak forces holding the molecules together (such as paraffins, which only have London van der Waals interactions) have low melting points, whereas crystals with strong lattices (i.e. those held together with strong attractive forces) have high melting points.
Crystals are produced by inducing a change from the liquid to the solid state. There are two options: one is to cool a molten sample to below the melting point. Pharmaceutical examples of crystallizing through cooling include the formation of suppositories, creams and semi-solid matrix oral dosage forms (although these will not always yield crystalline material). The other method of crystallization is to have a solution of the material and to change the system so that the solid is formed. At a given temperature and pressure, any solute (where the solute is the material that has been dissolved and the liquid is the solvent) has a certain maximum amount that can be dissolved in any liquid (called a saturated solution). If crystals are to be formed from a solution, it is necessary to have more solute present than can be dissolved, which is known as a supersaturated solution. As crystals form from a supersaturated solution, the systems will progress until there are solid particles in equilibrium with a saturated solution. In order to make a solid precipitate out of solution one can:
Many drugs are crystallized by adding water as an anti-solvent to a solution of the drug in an organic liquid. For example, if a drug is almost insoluble in water but freely soluble in ethanol, the drug could be crystallized by adding water to a near-saturated solution of the drug in ethanol.
The processes by which a crystal forms are called nucleation and growth. Nucleation is the formation of a small mass onto which a crystal can grow. Growth is the addition of more solute molecules onto the nucleation site. In order to achieve nucleation and growth, it is necessary to have a supersaturated solution. As mentioned above, a supersaturated solution is one where the amount of solute dissolved in the liquid is greater than the true solubility. Supersaturated solutions are not thermodynamically stable, so in these circumstances the system will adjust in order to move back to the true solubility, and to do this, the excess solute will precipitate. However, in some circumstances the process of nucleation can be slow. Many students will at some stage have had a supersaturated solution which has not crystallized but on simply scratching the side of the beaker with a glass rod, crystallization was induced. The scratching action produces a small amount of rough surface that acts as a nucleation site and causes the supersaturated solute to precipitate rapidly.
Polymorphism
If the crystallization conditions are changed in any way, it is possible that the molecules may start to form crystals with a different packing pattern to that which occurred when the original conditions were used. The change in conditions could be a different solvent, a change in the stirring, or different impurities being present. In Figure 8.1b, an alternative packing arrangement is shown to that which occurred for the same molecule in Figure 8.1a. As both the packing arrangements in Figure 8.1 are repeating ordered systems, they are both crystals; these would be called polymorphic forms.
By looking at the packing arrangements in Figure 8.1, it can be seen that the molecules in (a) are more spaced out than those in (b), which means that the two crystal forms would have different densities (i.e. the same mass of material would occupy different volumes). It looks as though it would be easier to physically pull a molecule off structure (a) than (b), as the molecules in (b) are more interwoven into the structure. If this were the case, then (a) would have a lower melting point than (b), and (a) may dissolve more easily. Also if an attempt were made to mill the two crystals, it looks like (a) would break easily, as there are natural break lines (either vertically or horizontally), whereas (b) does not seem to have an obvious weak line to allow easy breakage. This could mean that the milling and compaction (tableting) properties of the two forms will differ. In summary, a change in the packing arrangement of the same molecule, giving two different crystal forms, could result in significant changes in the properties of the solid.
Many organic molecules, including drugs and excipients, exhibit polymorphism. Often this is of a form called monotropic polymorphism, which means that only one polymorphic form is stable and any other polymorph that is formed will eventually convert to the stable form. However, some materials exhibit enantropic polymorphism, which means that under different conditions (temperature and pressure) the material can reversibly transform between alternative stable forms; this type of behaviour will not be considered further here. Considering monotropic polymorphism, the true stable form has the highest melting point and all other forms are described as metastable. This means that the other forms exist for a period of time, and thus appear stable, but given a chance they will convert to the true stable form. Different metastable forms can exist for very short times or many months before they convert to the stable form, depending upon the conditions under which they are stored.
In general, there will be a correlation between the melting point of the different polymorphs and the rate of dissolution, because the one with the lowest melting point will most easily give up molecules to dissolve, whereas the most stable form (highest melting point) will not give up molecules to the solvent so readily.
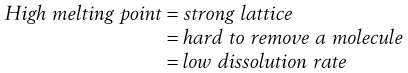
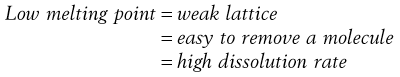
It is relatively easy to understand that changes in polymorphic form can cause changes in the rate at which a drug will dissolve. However, it is less easy to understand why this can lead to a change in the apparent solubility. Nonetheless, it is true that when a metastable polymorphic form is dissolved, it can give a greater amount of material in solution than the saturated solubility. In other words, metastable forms can dissolve to give supersaturated solutions. These supersaturated solutions will eventually return to the equilibrium solubility, due to the stable crystal form precipitating from solution, but that process may not be instantaneous. In fact, the supersaturated solution can often exist long enough to cause an increase in bioavailability of a poorly soluble drug. In Figure 8.2 the solubility of two different polymorphs of sulphamethoxydiazine is shown. It can be seen that Form II, a metastable form, has a higher solubility than Form III, a stable form, and that this lasts throughout the 90-minute experiment. However, if crystals of Form III are added to the solution of Form II then the solubility reverts rapidly to that of Form III, because the excess solute in the supersaturated solution will have seed crystals of Form III on which to precipitate.
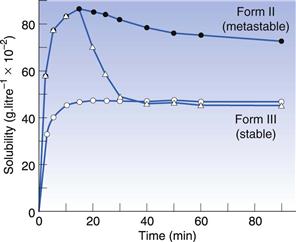
Fig. 8.2 The solubility time relationship for sulphamethoxydiazine. Open circles: solubility of polymorphic Form III, which rises to the drug’s equilibrium solubility and plateaux. Filled circles: solubility of polymorphic form II, which dissolves to twice the extent of Form III and then shows a gradual decline with time, as the stable form crystallizes from solution. Triangles: the effect of adding crystals of Form III to the solution of Form II at the peak of solubility. It can be seen that the amount dissolved falls rapidly from the supersaturated level to the true equilibrium solubility because the added crystals of Form III act as nucleation sites. Reproduced from Ebian et al 1973, with permission.
Polymorphism and bioavailability
Many drugs are hydrophobic and have very limited solubility in water. For drugs of this type, the rate at which they dissolve will be slow (slow dissolution rate), due to their limited aqueous solubility, and this can result in only a small percentage of the administered drug actually being available to the patient (low bioavailability). A classic example of the importance of polymorphism in bioavailability is that of chloramphenicol palmitate suspensions. In Figure 8.3 the blood serum level is plotted as a function of time after dosing. It can be seen that the stable α-polymorph produces low serum levels, whereas the metastable β-polymorph yields much higher serum levels when the same dose is administered.
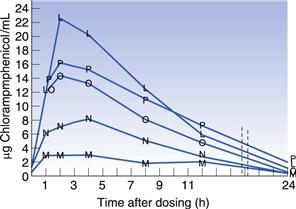
Fig. 8.3 Comparison of mean blood serum levels after administration of chloramphenicol palmitate suspensions using varying ratios of the stable (α) and the metastable (β) polymorphs. M = 100% α polymorph, N = 25 : 75 β:α, O = 50 : 50 β:α, P = 75 : 25 β:α, L = 100% β-polymorph. Reproduced from Aguiar et al 1976, with permission.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree