Parenteral drug delivery
Robert Lowe
Chapter contents
Reasons for choosing parenteral administration
Routes of parenteral administration
Intravenous injections and infusions
Intra-arterial and intracardiac injections
Category-specific requirements
Absorption from injection sites
Factors affecting absorption from the injection site
Key points
• Parenteral preparations are administered to a patient by injection.
• All parenteral products must be sterile.
• Parenteral preparations intended for multiple use must contain an antimicrobial preservative.
Introduction
In medicine and pharmacy, enteral administration is the term used to describe drug administration via the gastrointestinal tract. The majority of medicines are administered orally via this route in the form of tablets, capsules or liquids. The enteral route also encompasses rectal administration utilizing dosage forms such as suppositories, enemas or rectal ointment. In contrast to this, the term parenteral administration literally means any method of drug administration which does not utilize the gastrointestinal tract, such as by inhalation or application to the skin. In practice however, parenteral administration is commonly taken to mean drug administration by injection.
In this chapter, we will explore why the parenteral route of administration may be chosen by the clinician or the manufacturer of a medicine. The routes available for parenteral administration and the tissues, organs and anatomical spaces that can be accessed by injection are outlined. The various forms or types of parenteral product commonly manufactured are described and the pharmacopoeial standards for injectable products are discussed. The ingredients of formulated injectable products with regard to vehicles or solvents, excipients and preservatives are described along with physiological considerations, such as the pH and tonicity of the product prior to administration. Finally the containers, closures and primary packaging commonly used for parenteral products are discussed.
Reasons for choosing parenteral administration
The vast majority of patients would prefer to receive their medication as an oral tablet or liquid to swallow, or as a cream, ointment or transdermal patch to apply to the skin rather than receive treatment via injection, which can be painful or stressful (indeed some patients suffer from needle phobia). From a manufacturer’s point of view it is often simpler and much cheaper to prepare medicines such as tablets or liquids, particularly given the less stringent requirements for manufacturing premises for these non-sterile products, compared to the costs associated with manufacturing sterile medicines, such as injections, in highly specialized, controlled environments. There are, however, a number of clinical advantages associated with parenteral administration.
Many medicines are administered parenterally simply because the drug molecule itself would be rapidly broken down in the gastrointestinal tract and would thus become inactivated before it could be absorbed into the circulatory system. Good examples of this are aminoglycoside antibiotics, such as gentamicin. The injectable route may be chosen to provide a highly localized effect. This is particularly true when the injection route accesses a particular anatomical area or organ system. Examples of this include the injection of drugs, such as steroids, into joint spaces (intra-articular injection), intra-ocular injections to treat eye diseases or intrathecal injections where medicines are administered into the spinal column to deliver drugs into the cerebrospinal fluid, that otherwise might not accumulate sufficiently in this tissue to achieve the desired effect.
Intravenous injection delivers the drug directly into the circulatory system, where it is then rapidly distributed around the body. This is important clinically as the drug will rapidly produce an effect, whereas peak blood levels may not be achieved for one to two hours after a drug is administered orally. This rapid onset of action for an intravenously administered drug may be critical in emergency situations. Conversely, by choosing to administer a drug by intramuscular injection, the release of the medicine from the injection site into the circulation can be delayed and prolonged. Indeed, as will be seen later, by manipulation of the formulation of intramuscular injections it is possible to provide prolonged drug release allowing doses to be required at only once-monthly intervals. Finally, the intravenous route of injection is routinely used to administer medication to the unconscious patient who is unable to swallow. This route is also employed in conscious or unconscious patients if the gastrointestinal tract is not working. In this scenario, not only are medicines, but fluids for hydration and electrolyte replacement, plus all the nutrients, vitamins and trace elements normally obtained from a healthy diet supplied by parenteral nutrition provided intravenously.
Routes of parenteral administration
As noted above medicines are injected by many different routes and the choice of route is governed by the purpose of the treatment and the volume of medicine to be administered.
Intravenous injections and infusions
Intravenous (IV or i.v.) injections and infusions are administered into an easily accessible prominent vein near the surface of the skin, typically on the back of the hand or in the internal flexure of the elbow. The volumes administered can range from 1 mL for an intravenous injection, up to several litres for an intravenous infusion. Medicines administered by intravenous injection (or intravenous bolus dose) will rapidly increase the concentration of the drug in the plasma and produce a rapid effect. If the medicine is first added into a large volume of fluid (500 mL to 1 L infusion bag) and then administered by intravenous infusion at a slow and controlled rate, often utilising a pump, the drug will enter the circulation at a much slower and controlled rate. By altering the infusion rate it is possible for the clinician to titrate the dose against the effect required, e.g. controlling blood pressure, by manipulating the infusion rate of, for example, an inotropic drug such as dobutamine.
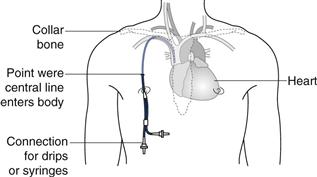
Drug solutions at high or low pH or highly concentrated hypertonic solutions (see below) will damage the cells lining the vein and cause localized pain and inflammation (thrombophlebitis). In order to avoid this problem a central line may be inserted. This is a long, indwelling catheter inserted into a vein in the neck or forearm with the end of the catheter sited in the superior vena cava close to the right atrium of the heart (see Fig. 36.1). Medicines administered intravenously, via a central line, become rapidly diluted in a large volume of blood and do not cause local irritation to the blood vessel. It is worth noting that injections which are formulated either as water in oil emulsions or suspensions must not be administered by the intravenous route. This is because the suspended drug particles can physically block blood capillaries and the oil phase of a water-in-oil injection could cause a fat embolism, again blocking blood vessels.
Intra-arterial and intracardiac injections
The large majority of drugs that are administered parenterally are given intravenously. As noted above, this delivers the drug directly into the blood stream to provide a rapid and predictable clinical effect. However, it is not the only way that medicines can be administered into the vascular system.
Intra-arterial administration is essentially the same as intravenous administration except that the drug is administered into an artery rather than a vein. Arteries are not as readily accessible as veins and this technique is much more invasive, and carries a greater risk than simple intravenous administration. For this reason it is seldom used. Intra-arterial administration is sometimes used when intravenous access cannot easily be established, such as in very premature infants, due to the very small size of their veins in relation to the catheter tubes used to maintain vascular access. Intra-arterial administration has also been used in the treatment of some cancers (such as liver cancer) where the anti-cancer medicines are injected into an artery upstream of the tumour site to ensure the maximum amount of drug reaches the tumour before distribution elsewhere around the body. However, the benefits of this method of administration do not appear to outweigh the risks to any significant degree.
Intracardiac injections are used to administer a drug (a common example being an aqueous solution of adrenaline) directly into either cardiac muscle or into a ventricle of the heart. This is undertaken only in life threatening emergencies to produce a rapid, local effect in the heart during a heart attack or in circulatory collapse.
Intradermal injections
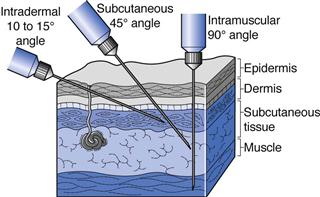
Intradermal (ID or i.d.) injections are given into the skin between the epidermal and dermal layers (Fig. 36.2). Volumes of up to 0.2 mL can be given by this route and absorption from the intradermal injection site is slow. This route is used for immunological diagnostic tests, such as allergy tests, or the injection of tuberculin protein to determine immunity against tuberculosis. Some vaccines such as BCG (tuberculosis) are administered by intradermal injection.
Subcutaneous injections
Subcutaneous (SC, s.c. also called hypodermic) injections are administered into the loose connective and adipose tissues immediately beneath the dermal skin layer (Fig. 36.2). Typical injection sites are the abdomen, upper arms and upper legs. Volumes of up to 1 mL can be administered comfortably, and aqueous solutions or suspensions of drugs are administered by this route. As this tissue is highly vascular, drugs administered by the subcutaneous route are fairly rapidly and predictably absorbed from this site. A common example of a drug administered by subcutaneous injection is insulin.
Intramuscular injections
Intramuscular (IM or i.m.) injections are preferably administered into the tissue of a relaxed muscle (Fig. 36.2). The muscle sites commonly used for intramuscular injection are the buttock, thigh or shoulder muscles. Aqueous or oily solutions or suspensions can be administered in volumes of up to 4 mL. In adults, the gluteal, or buttock, muscle will be used for larger volume injections, whereas in children the thigh muscle is usually larger and thus preferred. Drugs administered by the intramuscular route are more slowly absorbed from the injection site into the systemic circulation compared to those administered by the subcutaneous route.
Intraspinal injections
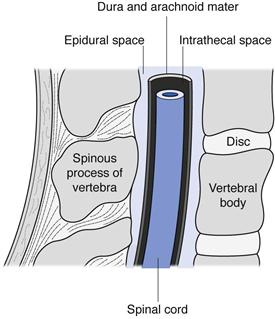
Intraspinal injections are given between the vertebrae of the spine into the area of the spinal column (Fig. 36.3). Only drugs in aqueous solution are administered by this route. Intrathecal (IT or i.t.) injections are administered into the cerebrospinal fluid (CSF) in the subarachnoid space between the arachnoid mater and the pia mater, the two innermost protective membranes surrounding the spinal cord. This route can be used for spinal anaesthesia, and in this case the specific gravity of the injection can be manipulated to localize the site of action and thus the area of the body anaesthetized. Intrathecal injections are also given to introduce drug substances into the CSF that would otherwise not diffuse across the blood brain barrier. Typically this could be antibiotics to treat meningitis or anticancer agents such as methotrexate or cytarabine. Volumes up to 10 mL can be administered by intrathecal injection.
Intracisternal injections are given between the atlas and axis vertebrae into the cisterna magna. This route is also used for antibiotic administration into the CSF, or to withdraw CSF for diagnostic purposes.
Epidural injections or infusions are given into the peridural space between the dura mater (the outermost protective membrane covering the spinal cord) and the vertebrae. This route is commonly used for spinal anaesthesia, for example during childbirth.
Intra-articular injections
Intra-articular (IA or i.a.) injections are given into the synovial fluid of joint cavities such as the knee. Aqueous solutions or suspensions can be administered by this route. This route of injection produces a local effect, and typically anti-inflammatory drugs are administered to treat arthritic conditions or sports injuries.
Ophthalmic injections
Ophthalmic injections are administered either around or into the eye; in the latter case these are referred to as intraocular injections (Chapter 41). Subconjunctival injections usually of 1 mL or less are administered under the conjunctiva or into the skin surrounding the eye (inside the eyelid for instance). Intraocular injections can be further classified as intracameral injections into the anterior chamber of the eye (in front of the lens), or intravitreal injections into the vitreous chamber (behind the lens). Intracameral injections can be from 0.1 mL to 1 mL in volume depending on whether the drug is left in the eye or administered during surgery on the open eye. This route has been used to administer antibiotics or local anaesthetics during eye surgery (e.g. cataract surgery). Intravitreal injections are used to administer a number of different drugs used to treat various ocular diseases. Because of the danger caused by raising intraocular pressure which can damage the retina, a maximum volume of only 0.1 mL can be administered by the intravitreal route. Ophthalmic drug delivery by injection is discussed further in Chapter 41.
Pharmacopoeial requirements
General requirements
Almost all pharmacopoeias lay down the requirements for parenteral products in a series of general monographs, with which all parenterally administered medicines are expected to comply. Most pharmacopoeias are very similar in their requirements, although details do differ and these need to be checked closely. Several different categories of parenteral preparation are described and further requirements specific to a given medicinal form are specified. This is discussed below.
Sterility
All parenteral preparations are sterile preparations intended for injection, infusion or implantation into the body. The requirement for sterility is vital as the method of administration of these products bypasses the body’s natural defence systems and barriers (such as the skin or gastrointestinal system), and introduces the medicine directly into the bloodstream or other body tissues. The methods of sterilization of parenteral medications are discussed in Chapters 16 and 17.
Excipients
Excipients may be added to parenteral preparations to serve a number of purposes. They can be added to make the preparation isotonic in relation to human blood, to adjust the pH, to increase the solubility of the drug substance, to increase the stability of the drug substance and increase the shelf-life of the product or to act as a preservative. The use of such excipients will be discussed more fully below. However it is worth noting here that the use of excipients should not adversely affect the action of the drug substance, or cause any side effects or toxicity at the concentrations used in a given formulation.