Soft Tissue Reconstruction with Flap Techniques
Luis O. Vasconez
Salman Ashruf
Franziska Huettner
Introduction
Sound understanding of reconstructive surgery is imperative to all practicing general surgeons. The integumentary system is the largest organ system of the body. It is subject to systemic disease as well as the extremes of the environment. The ability to surgically manipulate and reconstruct acquired defects in an efficacious fashion is the learning objective of this chapter.
In today’s medical environment the reconstructive ladder is no longer practical. Reconstructive surgeons must have a solid understanding of the surgical options available and those procedures that provide the best outcome, even though it may be the most complex solution.
In the management of large wounds created by extirpation of benign or malignant tumors, postradiation ulcerations, or posttraumatic defects, the cooperative efforts between the general surgeon and the plastic surgeon will most likely result in satisfactory coverage of the wound and a shorter recovery and hospitalization for the patient. In the occasions when the wound cannot be closed primarily or in cases where a skin graft will not suffice, the use of flaps is the most appropriate option. Different approaches are required in different clinical settings such as congenital defects, infected wounds, postradiation ulcers, as well as tumor excision. The coverage needed may be skin alone, or skin and fat, such as for reconstruction of the breast; muscle or omentum for the coverage of postradiation wounds or composite tissue such as skin and muscle; and, often, muscle and bone or any combination.
During the initial evaluation, it is essential to recognize the type of soft tissue defect with which one is dealing. For example, if it is a loss of the skin such as a large excision of a melanoma of the face, the defect can be covered by an adjacent skin flap. If one is dealing with a surgical loss of the breast, one needs to reconstruct by replacing the skin and the breast mound with either adipose tissue or, occasionally, the use of an implant. If, on the other hand, one is dealing with a postradiation persistent or recurrent tumor that requires a large extirpation, the defect needs to be covered with muscle or a musculocutaneous flap or by the use of omentum. In the case of postradiation wounds, although adjacent musculocutaneous flaps are given first priority, the use of a free flap should be considered. The essential principle for reconstructive soft tissue wounds is the application of the most effective flap that will succeed in covering the defect, regardless of the complexity of the procedure. Sufficient experience has determined what is more likely to succeed. One should forego the trial and error of using a simpler procedure that may not work and eventually end up with the appropriate but more complex flap reconstruction.
Skin grafts can include the entire epidermis and dermis, or the epidermis with a portion of the dermis. The former, known as a full-thickness graft, resists contraction, needs a well-vascularized recipient bed, maintains the adnexal structures, and has pigmentation similar to normal skin. Split-thickness skin grafts are more likely to contract or shrink, hyperpigment, and survive under less favorable conditions, and they leave less donor site morbidity. Factors that lead to skin grafts not “taking” include shear, hematoma, infection, and an avascular bed. For a skin graft to take requires a vascularized bed. These recipient sites can be muscle, fascia, paratenon, or periosteum. When such a bed is absent, a well-vascularized flap is necessary to provide durable coverage.
A flap is a unit of moveable tissue that remains attached to its original blood supply. Flaps can contain skin, muscle, bone, fascia, or any combination of these. When the flap contains a named septocutaneous or musculocutaneous blood vessel, it is known as an axial flap. It can therefore be transferred reliably to different sites to reconstruct soft tissue defects that may also require specialized tissue, such as fascia to restore abdominal wall integrity. Vascular anatomy of any particular flap is well known at this time and makes it reliable as long as one includes the vascular pedicle within the flap. We also know the safety of the length for that particular flap. The so-called random flaps (except for the face), where the distinct vascular anatomy is unclear, should be avoided because of the uncertainty of success. These flaps are perfused exclusively through the subdermal, or more accurately, the subcutaneous plexus and have no named blood vessel that supplies them. Similarly, so-called delayed procedures, which have the objective to improve the vascularity of a flap, are rarely if ever indicated because there is no assurance that the safety of the flap will be increased. The “delay” procedure also has the disadvantage of an additional operation and the concomitant swelling and edema that ensue, making it more difficult to transfer such a flap. A microvascular free flap, first reported in 1973, allows the transfer of tissues to distant sites with the reestablishment of blood flow to the flap by anastomosis of the artery and the vein to appropriate recipient vessels in the vicinity of the defect. The free flaps are most effective in providing coverage of areas with insufficient local, adjacent soft tissue. Performed by experienced microsurgeons with an appropriate team, the free flap is one of the safest of all flaps, with a success rate exceeding 95%.
General Principles for Coverage of Soft Tissue Wounds
Difficult wounds usually fall into three categories: (a) superficially infected wounds, (b) wounds over bone devoid of periosteum, and (c) postradiation wounds. We will describe principles for their management individually.
Superficially Infected Wounds
Chronic wounds in which granulation tissue has been allowed to develop and to persist for a prolonged time are often difficult to treat. Pathophysiologically, one should note that granulation tissue is a combination of capillaries, fibroblasts, and bacteria. Of these three elements, only one is helpful to the surgeon, the capillaries. Consequently, if a wound is filled with granulation tissue, it is essential that one resect surgically in a tangential way until all of the granulation tissue is removed and one gets down to a clean fascial level. Following such tangential resection, a simple meshed split-thickness skin graft may suffice, but in cases in which the wound cannot be completely cleansed or excised because that would entail the removal of essential structures, or in such patients with pressure sores, those infected wounds demand coverage with an adjacent muscle. Muscle has an antibacterial effect on the coverage of infected wounds, most likely because it brings in additional blood supply, which helps in the amelioration of the infection. The use of an adjacent fasciocutaneous flap is a second choice, although this is not as effective as muscle.
Wounds with Exposed Bone Devoid of Periosteum
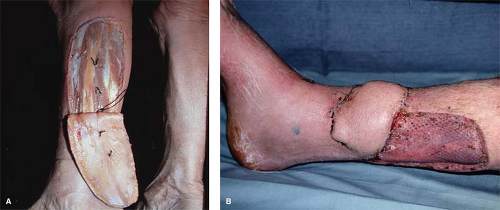
In these types of wounds, it is essential that one try to maintain the exposed bone moist with frequent dressing changes or a slow saline drip. In a recently traumatized extremity with exposed tibia, one could use an adjacent fasciocutaneous flap. However, when dealing with cases in which there is active or chronic infection, such as in chronic osteomyelitis, the treatment is much different. Following an adequate debridement of the wound, it is essential that one cover that defect immediately with an adjacent muscle to fill in the cavity and to cover the exposed bone. If the skull is exposed, as may occur following an electrical burn, it is not essential to remove the exposed skull but to cleanse it and to cover it with a free muscle flap, such as the latissimus dorsi. One has often rejoiced to see what appears to be dead bone revascularized or replenished once it is covered with well-vascularized muscle (Fig. 1).
Postradiation Wounds
The sophistication in the delivery of radiotherapy for the management of malignant tumors has resulted in the markedly decreased injury to the soft tissues, specifically the skin. We see much less ulceration or deformity following an appropriately delivered cancerocidal dose of 60 rads (radiation). However, we often see postradiation persistent and/or recurrent malignant tumors that must be salvaged with surgical
excision. If one is dealing with a persistent or recurrent tumor, the amount of excision should be left to the oncology surgeon. On the other hand, if one is dealing with a postradiation ulceration, a previous biopsy is not essential because the treatment is going to be the same, and the pathologist is likely to report: “Chronic ulceration with postradiation effects but tumor cannot be ruled out.” In either case, the surgical treatment of these postradiation problems consists of the extirpation not only of the tumor or the ulceration, but also of the entire portal of radiation. One determines the entire portal of radiation by noticing the slight hyperpigmentation of the skin, the lack of the skin appendages, as well as the leathery appearance and feeling of the skin.
excision. If one is dealing with a persistent or recurrent tumor, the amount of excision should be left to the oncology surgeon. On the other hand, if one is dealing with a postradiation ulceration, a previous biopsy is not essential because the treatment is going to be the same, and the pathologist is likely to report: “Chronic ulceration with postradiation effects but tumor cannot be ruled out.” In either case, the surgical treatment of these postradiation problems consists of the extirpation not only of the tumor or the ulceration, but also of the entire portal of radiation. One determines the entire portal of radiation by noticing the slight hyperpigmentation of the skin, the lack of the skin appendages, as well as the leathery appearance and feeling of the skin.
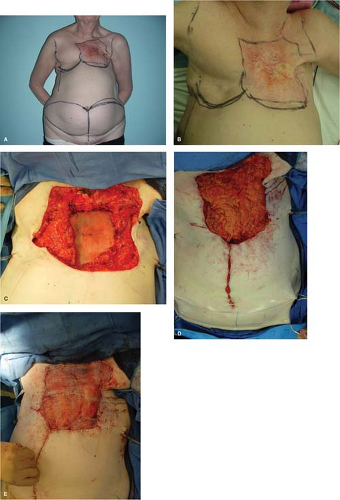
Following wide excision of the entire field that has been irradiated, one should immediately cover the defect with muscle or a musculocutaneous flap, and if that is not available, with omentum. A skin graft for postradiation wounds is not effective and is likely to fail. Adjacent skin flaps similarly may not adhere to the underlying postradiation bed or to the skin edges. Muscle or omentum is successful because of the increase in blood supply they bring to the wound. Over the muscle or the omentum, one can immediately place a meshed split-thickness skin graft. A transverse rectus abdominis muscle (TRAM) flap, either conventional or microvascular, is often successful if the skin edges to be approximated are beyond the field of radiation (Fig. 2).
Soft Tissue Flap Techniques
We will describe specific soft tissue reconstruction with flap techniques throughout the body that are likely to be encountered in a busy general surgical practice.
Facial Defects
Facial defects often result from excision of malignant tumors, whether simple basal cell carcinoma or more complex squamous cell lesions, and melanoma, which is increasingly seen today. We will describe specifically defects on the ear, nose, and cheek.
Defects of the Ear
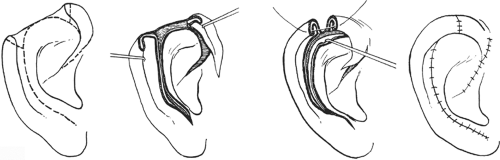
Removal of large basal or squamous cell carcinomas of the ear requires immediate reconstruction. If a wedge resection is so large that it may result in deformity of the ear, a local technique described by Antia is the more desirable method of reconstruction (Fig. 3). Following the excision of the large defect along the helix of the ear, the lower portion of the ear is incised along the helix through the skin and underlying cartilage to the ear lobe. If necessary, the upper portion similarly below the helix is also incised through the skin and the cartilage. This leaves the two ends of the helix able to be moved toward each other and to be approximated, thus restoring the normal helical contour of the ear. The inferior cartilage and overlying skin are also approximated.
This method of reconstruction has the advantage of restoring the normal shape of the ear and is relatively straightforward and effective. One does end up with a slightly smaller ear in comparison with the contralateral normal one, but this is not really a problem because we are seldom able to see both ears at the same time. A smaller, well-contoured ear is accepted by most casual observers.
Defects of the Nose
Basal cell carcinomas that occur along the dorsum or the tip of the nose often require flap coverage for a satisfactory result. The flap coverage should be from adjacent nasal skin because it provides the same color and texture as well as thickness, and in elderly patients, the scar is almost imperceptible. On the other hand, skin grafts may leave a visible concavity and a patch appearance because of the different color of the surrounding skin. We do not favor the routine use of the nasolabial flap for defects of the nose because it is a different skin from the nose, and
often leaves a “biscuit” type of appearance. For smaller defects, we advocate the use of the VY advancement flap or the banner flap. For total reconstruction of the nose, the midline forehead flap is safe and appropriate.
often leaves a “biscuit” type of appearance. For smaller defects, we advocate the use of the VY advancement flap or the banner flap. For total reconstruction of the nose, the midline forehead flap is safe and appropriate.
Vy Advancement Flap
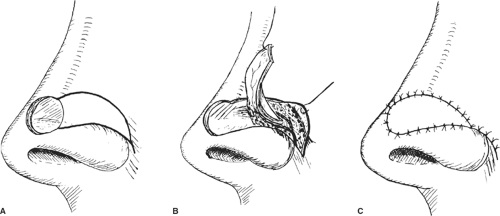
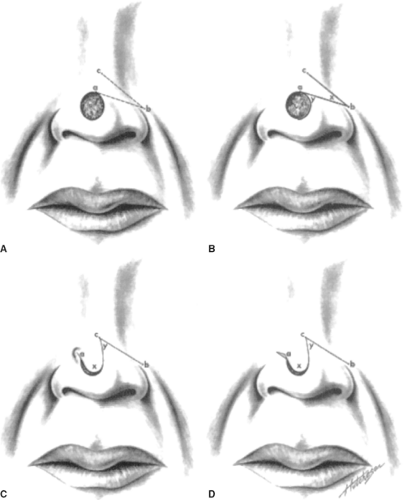
The VY advancement flaps rely on the mobility of the subcutaneous layer (Fig. 4). For smaller defects on the dorsum or the lateral aspect of the nose, once the lesion has been excised with appropriate margins, if it is malignant, one could design an adjacent VY flap that is incised and advanced to cover the defect. It is a simple and effective method for coverage of smaller defects.
Banner Flap
For larger defects, usually along the tip of the nose, a banner flap is indicated (Fig. 5). Again, the created defect is noted, and one outlines a transposition flap, which is elevated and transposed to cover the primary defect while the secondary defect is covered in the form of a Z plasty from the adjacent tissue along the ala. There may be a slight elevation of the involved ala, but this is usually minimally noticeable.
For larger defects along the tip of the nose where the banner flap will not suffice, it is advisable to use the entire remaining skin on the dorsum of the nose by outlining a flap that extends beyond the defect and along the lateral aspect of the nose to the glabella. By cutting it toward the opposite side by maintaining the angular vessel and freeing up the skin flap to cover the entire defect and the secondary defect along the glabella, it is closed in a VY type of fashion (Figs. 6 and 7). The dog ear that occurs can be handled at the same time by resection, thus making it a single-stage procedure.
Total Reconstruction of the Nose with a Midline Forehead Flap
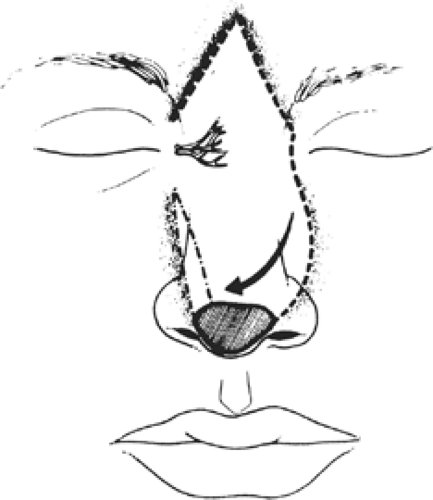
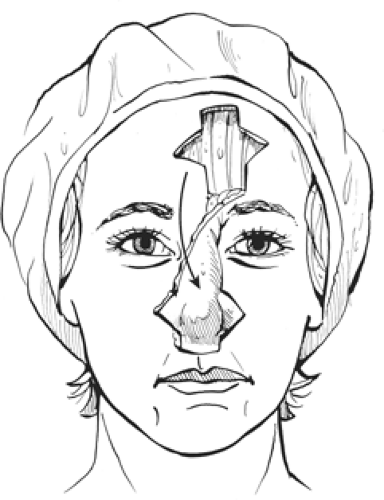
For patients with large basal or squamous cell carcinoma of the nose or postradiation persistent tumors, total excision of the overlying skin is indicated, and reconstruction, after appropriate margins have been obtained, is best done with a midline forehead flap (Fig. 8).
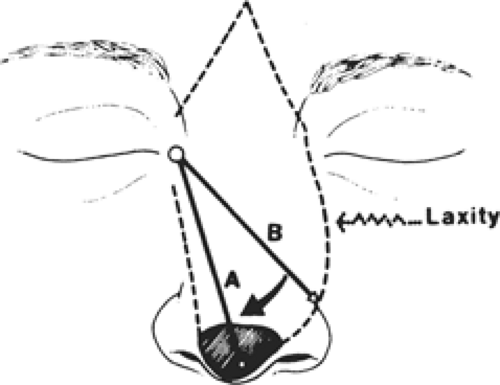
The techniques of the procedure are straightforward. A pattern is obtained of the area to be excised in the nose through transparent x-ray film and is transferred to the midline of the forehead. Following the excision of the tumor, the defect is confirmed again, and the dimensions of the flap are similarly drawn. The forehead flap is then incised and elevated at the subcutaneous tissue level to ∼2 cm above the glabella. At that point, the flap is deepened to include the galea to ensure that the blood supply, which comes from the angular vessels of the nose, is included. The flap is then
turned on either the left or the right axis, whichever is easiest to reach the distal portion of the defect. Although it is advisable to save at least one of the supratrochlear and supraorbital vessels, it is not essential because the midline forehead flap is supplied from the angular vessels of the nose that are located along the lower border of the nasal bones as it meets with the maxilla. The midline forehead flap does require a second stage, which is the division and insetting of the pedicle.
turned on either the left or the right axis, whichever is easiest to reach the distal portion of the defect. Although it is advisable to save at least one of the supratrochlear and supraorbital vessels, it is not essential because the midline forehead flap is supplied from the angular vessels of the nose that are located along the lower border of the nasal bones as it meets with the maxilla. The midline forehead flap does require a second stage, which is the division and insetting of the pedicle.
Breast Reconstruction
What was initially an imperfect product consisting only of the creation of a mound with an implant has now developed into a most satisfactory and acceptable method in which, following the extirpation of a breast, one is able to restore the removed structure and obtain symmetry with the opposite side. This is possible because of the introduction of the latissimus dorsi myocutaneous flap method of breast reconstruction in 1977, as well as the more sophisticated TRAM flap that was introduced in the early 1980s. Both methods have undergone considerable development and sophistication to increase their safety and versatility.
Most breast centers at the present time offer the patient different alternatives for treatment of breast cancer, and if the patient chooses mastectomy, immediate reconstruction is often suggested.
Immediate Versus Delayed Breast Reconstruction
It is our experience that patients who are offered immediate reconstruction following skin-sparing mastectomy accept it and are pleased with the eventual results. A considerable amount of literature has been published indicating the positive and favorable psychological effects of immediate breast reconstruction, particularly when it is done with relative simplicity, safety, and obtains symmetry with the opposite side. Questions that oncologists as well as patients ask and have been answered include: “Will reconstruction hide or delay the detection of recurrent cancer?” The answer is “No” because most of the recurrent cancers occur at the subcutaneous tissue level and alongside the mastectomy scar. This is easily detectable by palpation. Concerning the development of fat necrosis if a TRAM flap has been used, and which presents as a hardened mass, one can determine its exact nature by the rapidity of its appearance following the reconstruction (within weeks) and confirmation by fine-needle aspiration, which is read as fat necrosis. Will adjuvant therapy be delayed? No. Adjuvant chemotherapy is usually started 6 weeks following the mastectomy and reconstruction. In most cases, this is an appropriate schedule. In fact, studies have demonstrated that if there is no immediate breast reconstruction, the possibility of flap necrosis or seroma formation along the axilla often delays the initiation of chemotherapy beyond the 6-week interval.
Finally, patients ask whether they need blood transfusions. The answer is, most likely, no. Blood transfusions, although not proven deleterious for carcinoma of the breast as has been shown for carcinoma of the colon, are often not necessary even when one performs unilateral or bilateral TRAM flaps. This is because of the judicious use of lidocaine with epinephrine to elevate the flap, the judicious use of electrocautery for the undermining of the abdominal flap, and the realization that one does not need to transfuse unless the hematocrit falls below 20 or the patient is symptomatic.
Patients in whom immediate reconstruction would not be indicated include those with systemic diseases, which may make reconstruction more hazardous, such as patients on steroids or with uncontrolled diabetes. Similarly, if one knows preoperatively that the oncology surgeon plans to irradiate postoperatively, one would not perform an immediate reconstruction because, even if one uses a TRAM flap, it is likely to shrink and to become fibrotic. If one were to use an implant or an expander, failure as indicated by the exposure of the implant, infection, or inability to expand and to obtain symmetry with the other breast are very likely possibilities. For such cases, delayed reconstruction is necessary. In patients with large tumors who receive preoperative chemotherapy to shrink the tumor, immediate reconstruction is appropriate, although it is done with caution and with the expectation of problems with delayed healing, not only of the flap used for reconstruction, but also for the secondary defect, most likely in the abdominal wall, if the TRAM flap was used. The reason for this is that the deleterious effect of chemotherapy on wound healing persists for 6 to 12 weeks following the cessation of chemotherapy.
In patients with persistent or recurrent tumors following lumpectomy and radiation, immediate reconstruction is essential, either with a latissimus dorsi muscle or the TRAM flap because delayed wound healing or wound breakdown of the postradiation wound is likely to occur.
Nonetheless, a decreasing number of patients present months or even years later for delayed reconstruction. The aesthetic result is as satisfactory as with immediate reconstruction, although it has the disadvantage of requiring a second operative procedure and a second hospitalization.
Objectives and Principles of Breast Reconstruction
The objective of breast reconstruction is to perform a safe operative procedure that will obtain symmetry with the opposite breast. This often requires modifying the contralateral normal breast by either reduction mammoplasty or a mastopexy, which we advocate being done at the same time as the breast reconstruction.
The principles of breast reconstruction, which apply to any method used, include: (a) the preservation or restoration of the inframammary fold, (b) obtaining satisfactory projection, (c) obtaining satisfactory and matching ptosis with the contralateral breast, and (d) closing the axillary defect in immediate reconstructions (Fig. 9).
The Inframammary Fold
The inframammary fold is nothing more than the lower pole of the breast. Consequently, the oncology surgeon does not need to extend the dissection any further than the inframammary fold in completing the mastectomy. It is not unusual for some plastic surgeons to preoperatively place
some percutaneous sutures prior to the mastectomy. In a reconstructed breast with a TRAM flap, the inframammary fold is the lower end of the flap. However, if one has reconstructed the breast with the use of an implant or an expander, the inframammary fold represents the lower edge of the capsule around the implant. Because implants are likely to develop capsular contractures that raise the inframammary fold, most reconstructive surgeons place the expanders or the implants 2 to 3 cm below the inframammary fold so that the folds will be matched with the ensuing capsular contracture.
some percutaneous sutures prior to the mastectomy. In a reconstructed breast with a TRAM flap, the inframammary fold is the lower end of the flap. However, if one has reconstructed the breast with the use of an implant or an expander, the inframammary fold represents the lower edge of the capsule around the implant. Because implants are likely to develop capsular contractures that raise the inframammary fold, most reconstructive surgeons place the expanders or the implants 2 to 3 cm below the inframammary fold so that the folds will be matched with the ensuing capsular contracture.
Projection
Projection is defined as the transverse diameter from the sternum to the anterior axillary line. If one is to obtain more projection, if a transverse incision has been used for the mastectomy, the upper end of the incision needs to be zigzagged so that the transverse diameter will be larger. This is avoided with the use of the skin-sparing mastectomy because the entire envelope of skin is present except for the nipple and areola, and one need to fill only the skin envelope with tissue, usually the TRAM flap, to obtain a satisfactory projection.
Ptosis
Ptosis refers to a vertical dimension measured over a curved surface from the clavicle over the breast and down to the inframammary fold. If one has fixed the inframammary fold, either with sutures or preservation, one can place the flap in such a way that it will hang down below the inframammary fold to obtain ptosis. Usually this is done for a minimal amount of ptosis because it is preferable to modify the contralateral breast with a mastopexy or reduction mammoplasty. Ptosis is rarely possible with the use of an implant or an expander, except in the experienced hands of plastic surgeons who use oval or anatomic type of implants.
Closing the Axillary Defect
The fourth principle of breast reconstruction that should be addressed is closing the axillary defect. The breast extends only to the anterior axillary line. The oncology surgeon, even with a skin-sparing mastectomy, dissects the skin flaps to the anterior border of the latissimus dorsi muscle, which is in the posterior axillary line; the surgeon also will perform a sentinel node biopsy of the axilla. Consequently, it is essential that the reconstructive surgeon closes and obliterates the axillary defect by approximating the lateral skin flap to the anterior axillary line with sutures and placing a drain along the axilla, particularly in immediate breast reconstructions. In delayed breast reconstructions, the dissection of the flaps is done only to the anterior axillary line.
Bilateral Mastectomies
Presently, patients are electing to undergo bilateral mastectomies and immediate reconstruction. This usually entails doing the mastectomy and sentinel node biopsy on the side of the cancer, and usually a prophylactic skin sparing mastectomy on the contralateral side.
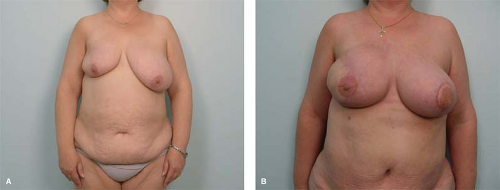
Patient who request bilateral mastectomies include not only those done for prophylaxis due to BRCA 1–2 gene, but also young patients who do not want to live with the burden of frequent checkups for recurrence of the cancer or the possibility of development cancer in the opposite breast. Other group who ask for bilateral mastectomies are those who have undergone previous lumpectomy, irradiation, and chemotherapy, and are seriously afraid of going through the suffering of possible radiation or chemotherapy in the future for cancer in the opposite breast (Fig. 10). A recent study by Boughey et al. indicates that contralateral prophylactic mastectomy in high-risk women with a personal history of breast cancer is associated with a survival advantage, reflecting an improved overall and disease-free survival. This might represent an additional reason for woman to decide for contralateral prophylactic mastectomy at the time of the cancer resection.
Options for Breast Reconstruction
There are several methods for breast reconstruction, and each one must be discussed
with the patient, addressing the advantages and disadvantages of each. The methods include the use of implants or expanders, the use of flaps such as the latissimus myocutaneous flap or the TRAM flap, and, in fewer instances, other flaps, including microvascular ones. These techniques vary considerably in their complexity. Symmetry may be more difficult to achieve with implants than with flaps.
with the patient, addressing the advantages and disadvantages of each. The methods include the use of implants or expanders, the use of flaps such as the latissimus myocutaneous flap or the TRAM flap, and, in fewer instances, other flaps, including microvascular ones. These techniques vary considerably in their complexity. Symmetry may be more difficult to achieve with implants than with flaps.
Implants and Expanders
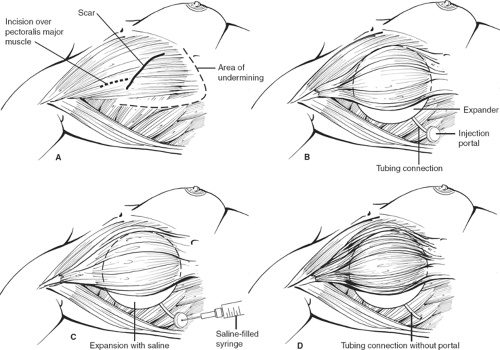
Patients who have good skin tone, are nonsmokers, have small breasts, and will not be receiving radiotherapy postoperatively are good candidates for implants. Most patients undergo placement of tissue expanders because one does not want to add an additional insult to the relatively thin overlying skin from underlying pressure. In addition, with mastectomies (except skin-sparing mastectomies), skin is removed and skin expansion is required.
The tissue expander or implant can be used for immediate or delayed reconstruction (Figs. 11 and 12). The prosthesis is placed submuscularly beneath the pectoralis major muscle and the serratus anterior muscle, if possible, to provide an extra layer of tissue in the event of a skin breakdown. The port is placed at the lower axilla, although some expanders have a built-in port that is located anteriorly and identified with a magnet. The hospitalization is short, 1 or 2 days, and the expansion is usually begun on the second week and proceeds at weekly intervals for a period of 4 to 6 weeks, depending on the amount of expansion required and how much the patient can tolerate each time. After the expansion is complete, the expander is left in place for <3 months in an effort to avoid the “recall phenomena,” thus allowing the expander capsule to mature so that contraction of the pocket does not occur once the expander is removed. The expander is replaced with a permanent prosthesis, and the port is then removed on an outpatient basis.
Advantages
Short operating time
Short recovery time
Lack of donor site scars
Lack of donor site morbidity
Disadvantages
The breast is only partly reconstructed and requires an expansion.
The reconstructed breast is round and creates unnatural upper pole fullness; to obtain symmetry, it is not unusual that one needs to put a smaller implant on the contralateral normal breast.
The reconstructed breast has an unnatural feel.
Potential complications are infection, capsular contracture, deflation, and extrusion. Capsular contracture may cause variable amounts of pain and an unnatural appearance of the breast. The incidence of capsular contracture has been reduced with submuscular placement of the implant. Capsulotomy or capsulectomy may be required for capsular contracture. Removal of the implant and autologous reconstruction with vascularized tissue may ultimately be required.
Additional Considerations
Although the use of expanders indicate a two-stage procedure, large centers indicate that there is, in fact, an average of 3.2 operations per patient because of the problems with exposure of the implant, capsular contracture requiring reoperation, elevation of the inframammary fold, as well as inadequate symmetry with the contralateral breast.
A second and most important consideration is the increasing use of skin-sparing mastectomy by oncology surgeons. This procedure develops thin skin flaps, which in most large series have an incidence of 18% for skin necrosis. Were one to put an implant or an
expander under this relatively thin skin, exposure of the implant would be unavoidable. To avoid exposure, total muscle coverage of the implant is essential. Although the serratus muscle does provide a certain amount of muscle coverage to the lower pole of the implant, if one depends only on the serratus muscle for such coverage, one is likely to place the implant higher than one would like, thus elevating the inframammary fold. Because of this consideration, in our practice when a skin-sparing mastectomy has been performed and an implant reconstruction is planned, we prefer to transpose the latissimus dorsi myocutaneous flap. This provides excellent muscle coverage for the lower portion of the implant and restores the skin island at the site of the nipple and areola. If this is done, as demonstrated in our practice, one does not need to put an expander; instead, a permanent implant is used, thus reducing the number of operative procedures. Even if the overlying skin flap were to necrose in a small portion, the implant is covered with muscle and is salvaged.
expander under this relatively thin skin, exposure of the implant would be unavoidable. To avoid exposure, total muscle coverage of the implant is essential. Although the serratus muscle does provide a certain amount of muscle coverage to the lower pole of the implant, if one depends only on the serratus muscle for such coverage, one is likely to place the implant higher than one would like, thus elevating the inframammary fold. Because of this consideration, in our practice when a skin-sparing mastectomy has been performed and an implant reconstruction is planned, we prefer to transpose the latissimus dorsi myocutaneous flap. This provides excellent muscle coverage for the lower portion of the implant and restores the skin island at the site of the nipple and areola. If this is done, as demonstrated in our practice, one does not need to put an expander; instead, a permanent implant is used, thus reducing the number of operative procedures. Even if the overlying skin flap were to necrose in a small portion, the implant is covered with muscle and is salvaged.
Use of Acellular Dermis and Biologic Mesh
Following a skin sparing mastectomy, it is not possible to cover the lower portion of an implant, if that is the method chosen by the reconstructive surgeon. We advocate lower coverage of the implant with the latissimus dorsi muscle, although this entails a bigger operation and turning of the patient. To avoid that, one has the option of using acellular dermis. These materials come in two general classes: human collagen and pig collagen (Alloderm and Strattice).
The materials although helpful to provide coverage of the implant or reinforce the thin skin flaps are devoid of deleterious effects. These effects may include the following: There is a low level of inflammation of the wound, which may be mistaken for an infection. This is due to the fact that collagen has a low level of antigenicity (perhaps more with the xenographic mesh—pig). There is invariable fluid collection or seroma, which requires the use of drains for a prolonged period of time. In addition, one has to be sure that the native skin flaps are well vascularized to avoid exposure of the mesh. Optimistic reports have been published, although generalized experience is limited. Nonetheless, it is an attractive and easy option, although the materials are quite costly.
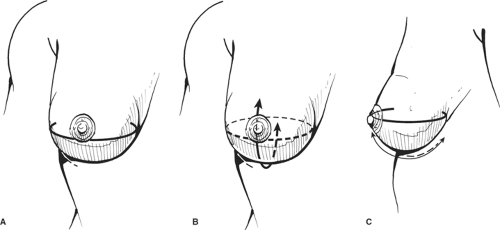
Latissimus Dorsi Myocutaneous Flap and Implant
This procedure is the safest method of breast reconstruction; it is relatively straightforward and very well tolerated by the patient. This technique is particularly applicable to immediate reconstruction following skin-sparing mastectomy. The latissimus provides additional muscle coverage to the implant, particularly on the inferior pole.
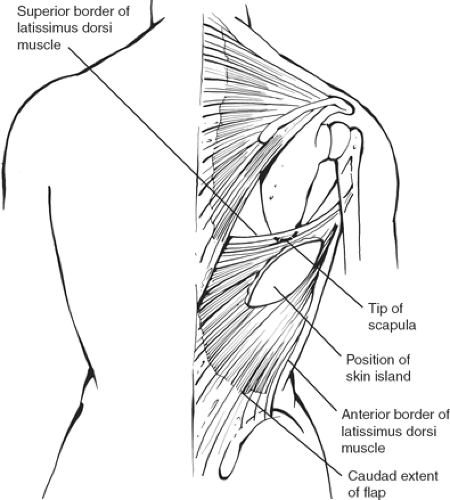
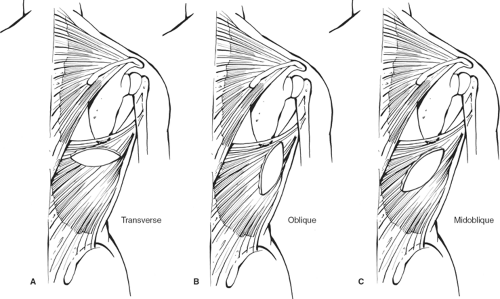
The latissimus dorsi is a rhomboid-shaped muscle that originates along the paravertebral fascia in the back and inserts in the upper portion of the humerus. The upper border of the muscle is just above the tip of the scapula, extending obliquely posteriorly, and inferiorly it then reaches the iliac crest (Fig. 13). It is a “tree-climbing” muscle, but its function can be taken over by other adjacent muscles. The blood supply is from the thoracodorsal artery, which is a branch of the subscapular artery (third portion of the axillary artery). The pivot point should be as high as possible, limited only by the thoracodorsal pedicle. The insertion need not be transected, but the muscle should be freed up as high as possible to make it transpose as a pendulum rather than rotating it to avoid the bulge along the axilla. The flap is usually harvested as a musculocutaneous flap with a skin island taken transversely across the back so that the scar will be covered with a brassiere. A skin island should be limited to no more than 6 to 8 cm to allow primary closure of the donor site.
Technique
The patient is marked in the standing position preoperatively. The anterior border of the latissimus dorsi muscle should be marked on the ipsilateral side, and the inframammary fold should also be marked. The skin paddle is oriented transversely to correspond to the brassiere line. If necessary, an extension along the anterior border of the latissimus dorsi muscle is done to gain access to the upper portion of the muscle.
Working in concert with the oncology surgeon, the flap can be elevated before the mastectomy or following it. If it is elevated before the mastectomy, the patient is placed on a bean bag in the lateral recumbent position, the flap, which is elevated and ready to be transposed, is placed on a sterile plastic bag, and the wound is closed with the insertion of drains. If the mastectomy is done first, the patient is placed in the lateral decubitus position with the shoulder and arm resting at 90 degrees over a Mayo tray. The skin island is incised right down to the muscle. The skin flap is elevated superiorly above the tip of the scapula and inferiorly for at least 10 to 15 cm. The dissection of the muscle is begun superiorly, where the upper border of the latissimus dorsi is easily identified, and extends posteriorly along the paravertebral fascia down the inferior border of the dissection while dividing the muscular attachments with the cautery. The anterior border of the latissimus dorsi is identified, and one grasps the muscle edges with an Allis clamp, and the elevation is continued by dividing the posterior intercostal attachments.
Care must be exercised to avoid elevating the serratus muscle. This is avoided by beginning the dissection superiorly where one finds the appropriate plane within the planned dissection. The muscle with the overlying skin island is freed up all the way around. The thoracodorsal vessel is easily identified in the axilla. Rarely, the serratus branch needs to be divided to improve the arc of rotation, but this is not routinely done. The thoracodorsal vessel does not need to be skeletonized, but the muscle should be freed up posteriorly, almost all the way to the humerus. The latissimus myocutaneous flap is then tunneled to the anterior chest by making a very high tunnel through the skin-sparing mastectomy dissection or by using the axillary incision that may have been made for the sentinel node biopsy. Variations of the flap may be used instead (Fig. 14).
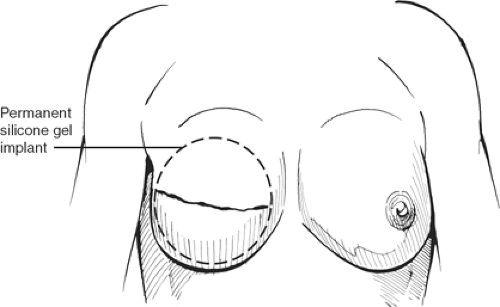
Once the myocutaneous unit is transposed anteriorly, one elevates the pectoralis major muscle, partially divides its origin along the sternum, and the latissimus dorsi muscle is sutured to the undersurface of the pectoralis major muscle and to the subcutaneous tissue of the skin in the inferior flap, in preference of suturing to the chest wall. An immediate implant is inserted and inflated to the proper volume, but it is advisable that the implant be placed at least 2 to 3 cm below the contralateral inframammary fold (Fig. 15). The reason for suturing the lower portion of the latissimus dorsi muscle to the subcutaneous tissue is to avoid restricting the implant pocket. A suction catheter is placed along the axilla.
The patient is then returned to the recumbent position, is sat up on the operating table, and appropriate adjustments are made if necessary by putting another smaller implant on the contralateral side.
Advantages
The implant is totally covered by muscle, and thus protected of the overlying soft tissue.
Disadvantages
Because an implant is used, capsular contracture remains a possibility even though there is total muscle coverage. Deflation is also possible, although extrusion is rare.
There is a donor site scar on the back, which usually is covered by the brassiere.
Seroma is very common at the donor site and is usually treated by a secondary stab wound percutaneous needle aspiration in the most dependent portion of the seroma.
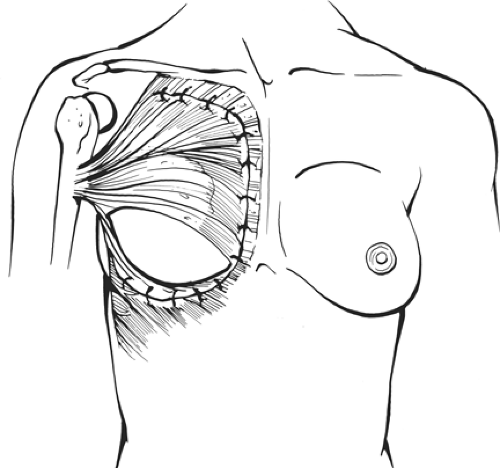 Fig. 15. Most often the distal third of the implant is not covered by muscle, although occasionally the serratus muscle does help achieve lower coverage of the implant. (Modified from Vasconez LO, Lejeur M, Gamboa-Bobadilla M. Atlas of Breast Reconstruction. Philadelphia: JB Lippincott, 1991:3.9, with permission.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|