INTRODUCTION
WHAT WE DO
Although malignant neoplasms of soft tissue are relatively uncommon, accounting for about 0.6% of all cancer deaths in the United States in 2010, soft tissue pathology is often considered as an overwhelming and intimidating area. Soft tissue pathology includes a wide spectrum of tumors from several supportive nonepithelial tissue types mostly of mesodermal origin (adipose tissue, smooth muscle, skeletal muscle, etc.) and the peripheral nervous tissue of neuroectodermal origin. Additionally, there are a significant proportion of tumors that cannot be classified beyond the generic term “sarcoma” despite the use of immunohistochemical tools, cytogenetic techniques, and molecular studies. The simplest way to approach soft tissue pathology is to divide the tumors into groups based on the line of differentiation of the neoplastic population. Therefore, this chapter will systematically explore the different types of soft tissue neoplasms based on lineage. Within each subset of tumors (lipomatous, smooth muscle, nerve sheath, etc.), there is a certain set of rules or principles that provide an approach to diagnostic workup. An understanding of these rules helps the pathologist to navigate through each group of tumors. The overall approach to the analysis of soft tissue masses will start with imaging studies most often using CT or MRI technology to define the location and extent of tumor and assay for the presence of potential metastatic lesions. Early biopsy using fine-needle aspiration, core needle, or open incisional biopsy will provide tissue to allow classification, histological grading, and often cytogenetic or molecular analysis. The study of chromosomal abnormalities is of particular importance in the classification of certain soft tissue tumors. For example, about 95% of Ewing sarcomas (ES) (see below) have a translocation involving the EWS gene on 22q12.
LIPOMATOUS TUMORS
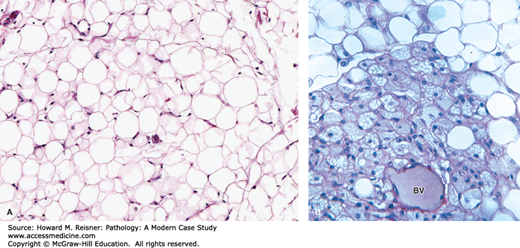
Adipose tissue can be divided in to white fat and brown fat. White fat consists of spherical adipocytes containing a single lipid vacuole that compresses a crescent-shaped nucleus at the periphery of the cell (Figure 18-1A). White fat provides several key functions such as thermal insulation, mechanical protection, and storage and release of lipid/free fatty acids in response to physiologic stimuli. Brown fat has the primary function of heat production and is typically found during the neonatal period in areas such as the axilla, perirenal region, and posterior neck. The abundant mitochondria in brown fat cells along with tissue vascularity are responsible for the red-brown coloration of this tissue. Microscopically, brown fat consists of a mixture of multivaculoated, granular cells with centrally placed nuclei and univacuolated cells that appear similar to cells of white fat (Figure 18-1B).
FIGURE 18-1
(A) White fat. Note single lipid vesicles and peripheral nuclei. (B) Brown fat. Note the multivacuolated nature of the cells and the centrally placed nuclei. BV indicates a small blood vessel. (Modified from Mescher AL, Junqueira’s Basic Histology Text and Atlas 12th edn. McGraw Hill Lange New York 2010, Figure 6-4A, page 113).
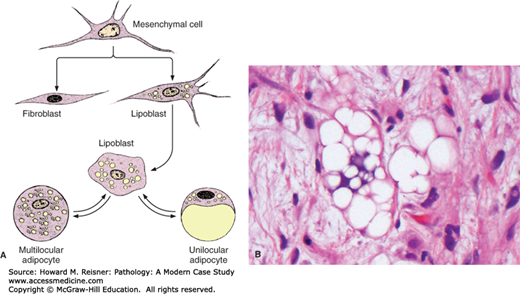
Lipoblasts are embryonic mesenchymal cells that are the center of much controversy in the topic of lipomatous neoplasms. Morphologically, these cells have a hyperchromatic-scalloped nucleus that is multiply indented by numerous fat vacuoles or indented by a single large fat vacuole. It is critical to appreciate that lipoblasts can be seen in both benign and malignant fatty tumors and the identification of lipoblasts is not always requisite for the diagnosis of liposarcoma (Figure 18-2).
FIGURE 18-2
Development of white and brown fat cells (A). Undifferentiated mesenchymal cells differentiate as preadipocytes and are transformed into lipoblasts as they accumulate fat and thus give rise to mature fat cells. The mature fat cell is larger than that shown here in relation to the other cell types. Undifferentiated mesenchymal cells also give rise to a variety of other cell types, including fibroblasts. When a large amount of lipid is mobilized by the body, mature unilocular fat cells may return to the lipoblast stage. (B) Lipoblast demonstrating central scalloped nucleus and numerous fat vesicles. (Figure 2a source from Mescher AL, Junqueira’s Basic Histology Text and Atlas 12th edn. McGraw Hill Lange New York 2010, Figure 6-3, page 112.)
QUICK REVIEW Rules for Lipomatous Neoplasms
Location, location, and location. Superficial (dermal, subcutaneous) lipomatous tumors are usually benign, while deep-seated (subfacial, intramuscular, retroperitoneal) fatty lesions are worrisome.
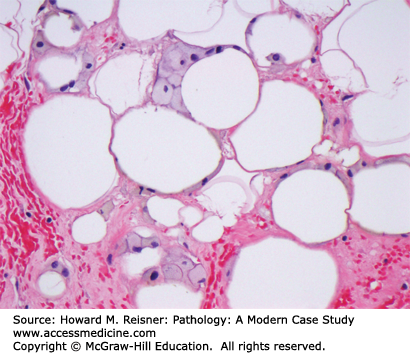
The three morphologic cell types/patterns that you should be able to recognize are fat necrosis (Figure 18-3), atypical hyperchromatic stromal cells (Figure 18-7), and lipoblasts (Figure 18-2B).
Almost all fatty neoplasms have described cytogenetic abnormalities. Fluorescence in situ hybridization for MDM2 gene amplification in atypical lipomatous tumors/well-differentiated liposarcoma (ALTs/WDL) and rearrangements of CHOP (DDIT3) in myxoid liposarcoma are often critical in the workup of these entities.
The most common soft tissue tumors are of lipomatous differentiation. Lipomatous tumors occur in both superficial and deep soft tissue. Location (subcutaneous, intramuscular, retroperitoneal) is often helpful in making the correct diagnosis. While immunohistochemistry is frequently used to elucidate a line of differentiation in other soft tissue tumors, the use of immunohistochemistry is limited in fatty neoplasms. Instead, ancillary studies such as cytogenetics and molecular techniques are invaluable in the categorization and diagnosis of some of these lesions. Distinct cytogenetic aberrations have been described in almost all fatty tumors.
CASE 18-1
A 40 year-old male presents with a 15 cm thigh mass.
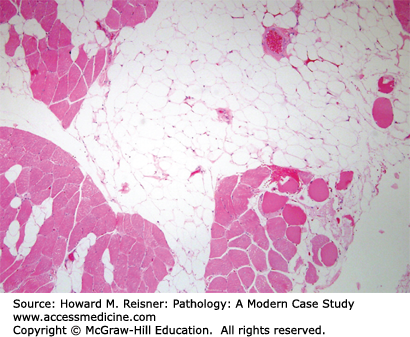
Cytogenetic studies revealed a translocation involving chromosome 3 and 12. No supernumerary ring or giant marker chromosomes were identified. Morphologic features, coupled with the cytogenetic findings, support the diagnosis of intramuscular lipoma. The case demonstrates the importance of cytogenetic analysis in differentiating between benign, and malignant lesions of adipocytic lineage (Figure 18-4).
BENIGN ADIPOCYTIC TUMORS
Lipomas are the most common soft tissue tumor and rarely are clinically significant. They typically present as a slowly growing painless subcutaneous mass in anatomic locations such as the back, shoulder, neck, abdomen, or proximal extremities. They may become large but average 3 cm in size. When superficial they are generally well circumscribed, but lipomas can have a diffuse pattern of growth if deep seated. Morphologically, lipomas consist of mature adipose tissue and have the gross yellow appearance of fat. Although these lesions are well vascularized, the blood vessels are usually compressed by the adipocytes and relatively inconspicuous. While thin fibrous bands may be identified, these septae are hypocellular without atypical cells. Up to two-thirds of lipomatous tumors show cytogenetic abnormalities, with aberrations involving 12q13-15 being most common. Lipomas only rarely recur after excision.
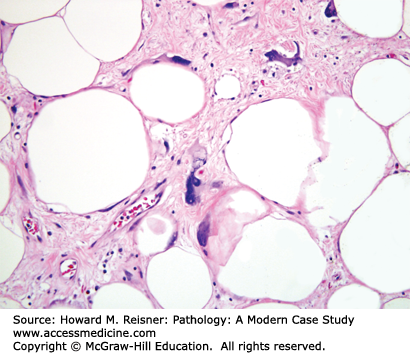
Variant forms of benign lipomas include angiolipomas, spindle cell/pleomorphic lipoma, and intramuscular lipomas. Angiolipomas are benign lipomatous neoplasms with unique clinical and morphologic features. The classic clinical presentation is a young adult male with multiple tender subcutaneous nodules. Morphologically, these tumors are composed of mature adipose tissue and clusters of capillary-sized vessels most easily recognized at the periphery of the lesion. Thrombi are seen in the lumens of the intralesional vessels (Figure 18-5). This type of lipomatous tumor is unusual because although familial cases have been reported, no distinct cytogenetic aberration has been characterized to date.
Spindle cell/pleomorphic lipoma is another benign lesion with a characteristic clinical history: an older male with posterior neck/back mass. In fact, with that clinical history, spindle cell lipoma should always be the first thing on the differential diagnosis. These tumors are well-circumscribed subcutaneous lesions that are composed of three elements: mature adipose tissue, bland spindle cells, and ropey collagen. Occasionally, multinucleated floret-like giant cells may be a component of these tumors (Figure 18-6). The spindled cells of spindle cell/pleomorphic lipoma are strongly CD34 positive, the one exception to the teaching that immunohistochemistry is not useful in fatty tumors.
Intramuscular lipomas are benign fatty tumors that occur within skeletal muscle of the extremities. Therefore, these tumors are deep-seated and are of clinical concern. Intramuscular lipomas rarely recur, and complete resection is usually curative (see Case 18-1).
MALIGNANT ADIPOCYTIC TUMORS
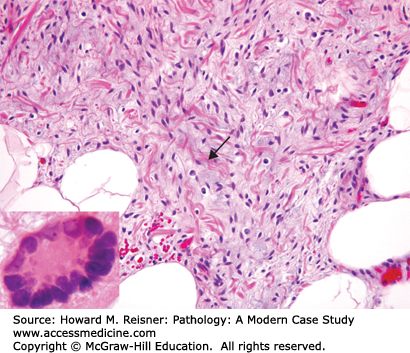
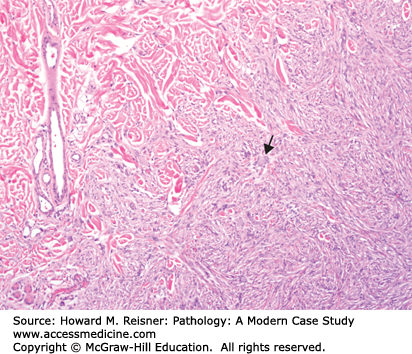
In clinical practice, when a pathologist examines a deep (intramuscular, retroperitoneal) well-differentiated lipomatous tumor, the key differential diagnosis is lipoma (a benign lesion) versus ALT/WDL (Table 18-1). The terms “atypical lipomatous tumor” and “well-differentiated liposarcoma” are best thought of as synonyms describing the same entity. The term ALT was introduced to describe lesions occurring in the extremities since those tumors almost always have a favorable prognosis. In contrast, those tumors located in the retroperitoneum have a worse clinical course with frequent recurrences and even death. There are several gross, microscopic, and cytogenetic/molecular features that can be helpful in making the distinction between lipoma and ALT/WDL. First, lipomas are typically grossly homogeneous, whereas examination of ALT/WDL may reveal fibrous bands. Second, ALT/WDL is characterized histologically by atypical hyperchromatic stromal cells that exhibit large, hyperchromatic smudgy nuclei. Such cells are often found in fibrous septae and vessel walls (Figure 18-7). It is the identification of these cells, not lipoblasts, which solidify the diagnosis of ALT/WDL. As alluded to earlier, ALT/WDL can recur and cause death. Those tumors located on the extremity recur in approximately 40% of cases, while retroperitoneal lesions have a recurrence rate of over 90%. ALT/WDL can recur but do not metastasize. However, with time, these tumors are at risk of dedifferentiation, and dedifferentiation occurs more frequently with retroperitoneal lesions than with those tumors involving the extremities. Morphologically, dedifferentiated liposarcoma is characterized by well-differentiated liposarcoma immediately adjacent to a high-grade nonlipogenic sarcoma, usually with an abrupt transition (Figure 18-8) Cytogenetic analysis has shown that these tumors contain the same abnormalities as well-differentiated liposarcoma (giant marker and ring chromosomes) but also usually have additional abnormalities.
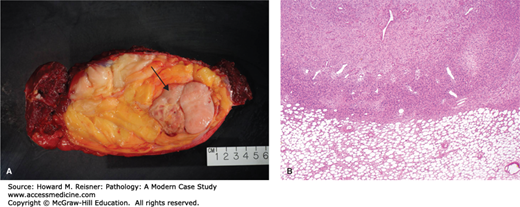
FIGURE 18-8
High-grade nonlipogenic sarcoma within a well-differentiated liposarcoma. (A) Gross surgical specimen showing tan area of “dedifferentiation” within the yellow lipogenic tumor (arrow). (B) Histology specimen shows abrupt transition between lipogenic well-differentiated area of tumor (lower third) and “dedifferentiated” high-grade nonlipogenic sarcoma (upper two thirds).
| Lipoma | Atypical Lipomatous Tumor/Well-Differentiated Liposarcoma | |
|---|---|---|
| Age | 40–60 years of age | Middle-aged to elderly |
| Site | Superficial (subcutaneous), occasionally intramuscular | Deep (intramuscular or retroperitoneal including groin) |
| Morphology | Mature adipose tissue No atypical hyperchromatic stromal cells | Mature adipose Fibrous septae Atypical hyperchromatic stromal cells (can be focal) |
| Cytogenetics | Translocation involving 12q13–15 | Supernumerary ring and giant marker chromosomes with amplified sequences of 12q14-15 region |
| Molecular | No amplification of MDM2 | MDM2 amplification |
| Behavior | Benign, only rarely recur | Extremities: recur, rarely dedifferentiate Retroperitoneum: frequently recur; up to 20% dedifferentiate |
Myxoid liposarcoma is another malignant lipomatous neoplasm. This tumor commonly presents in young to middle-aged adults in deep soft tissue of the extremities. Histologically, myxoid liposarcoma is characterized by round to stellate-shaped cells in a myxoid background with a prominent plexiform capillary network. Signet ring and less commonly multi-vacuolated lipoblasts can be identified in these tumors. Additionally, foci of “round cell differentiation” can be seen. These areas are composed of sheets of virtually back-to-back round cells with vascularity that is obscured by cellularity (Figure 18-9).
IN TRANSLATION
Fusion between the CHOP (DDIT3) gene on chromosome 12 and the FUS gene on chromosome 16 is sensitive and specific for the diagnosis of myxoid liposarcoma, and this finding can be helpful on small biopsy specimens when the diagnosis is in doubt. Myxoid liposarcoma can recur and metastasize, and tumors with >5% round cell differentiation have a worse prognosis.
WHAT WE DO Current Trends: Fluorescence in Situ Hybridization (FISH) for MDM2 Gene Amplification
Ancillary techniques utilizing cytogenetic and molecular tools can be critical in arriving at the correct diagnosis (Table 18-2). Frequently, atypical hyperchromatic cells are only found focally, and they may be missed due to chance or poor sampling. Therefore, the finding of supernumerary ring or marker chromosomes with cytogenetic studies or MDM2 gene amplification with fluorescence in situ hybridization is virtually diagnostic of ALT/WDL. In deep-seated lesions that are clinically worrisome but where histologic review fails to reveal cytologic atypia or in small biopsy samplings, cytogenetic and molecular tools are invaluable.
| Tumor | Cytogenetic Finding |
|---|---|
| Lipoma | Aberration of 12q13-15 |
| Spindle cell/pleomorphic lipoma | Deletion of 16q and 13q |
| Hibernoma | Rearrangement of 11q13-21 in several cases |
| Lipoblastoma | Rearrangement of 8q11-13 |
| Angiolipoma | Normal karyotype |
| Atypical lipomatous tumor/well-differentiated liposarcoma | Supernumerary ring and giant marker chromosomes with amplified sequences of 12q14-15 region |
| Myxoid liposarcoma | t(12;16)(q13;p11) |
VASCULAR TUMORS
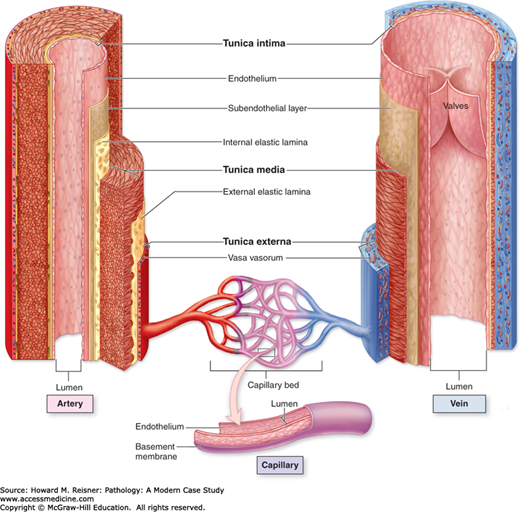
The vascular system consists of a series of vessels through which blood flows and can be divided into arterial (arteries, arterioles) and venous (veins, venules) systems. The arterial system is responsible for delivering blood pumped from the heart to capillary beds that are the site of gas exchange. Blood is returned to the heart via the venous system. Most vessels in the vascular system have the same basic structure: tunica intima, tunica media, and tunica adventitia. The tunica intima consists of a flattened endothelial layer surrounded by a basement membrane. The tunica media is an intermediate muscular layer and exhibits the most variation in arterial and venous components. The outer supporting layer is called the tunica adventitia. Capillaries consist only of a single layer of endothelial cells without muscular or advential components. Besides serving as a conduit for blood delivery, blood vessels play a role in coagulation and immune function (Figure 18-10).
FIGURE 18-10
Walls of arteries, veins, and capillaries. Walls of both arteries and veins have a tunica intima, tunica media, and tunica externa (or adventitia), which correspond roughly to the heart’s endocardium, myocardium, and epicardium. An artery has a thicker tunica media and relatively narrow lumen. A vein has a larger lumen and its tunica externa is the thickest layer. The tunica intima of veins is often folded to form valves. Capillaries have only an endothelium, with no subendothelial layer or other tunics. (From Mescher AL, Junqueira’s Basic Histology Text and Atlas 12th edn. McGraw Hill Lange New York 2010, Figure 11-7, page 190.)
The lymphatics are a unidirectional system of vessels and transport excess fluid from the interstitium to regional lymph nodes and finally to the venous system. Lymphatic vessels are found in almost all tissues, and the structures of lymphatics are similar to that of vessels of the venous system. In fact, it is often not possible to distinguish between veins and lymphatics on hematoxylin and eosin (H&E) sections.
CASE 18-2
A 10-year-old male presents with forehead lesion.
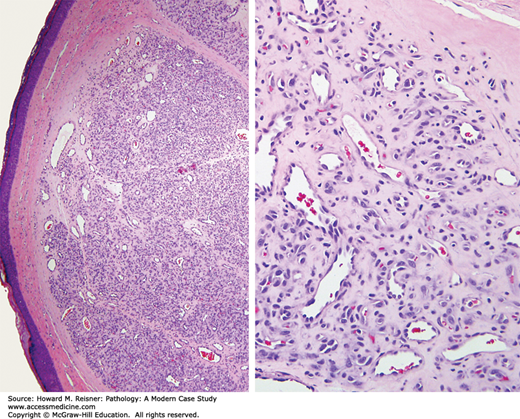
Histologic examination reveals a well-circumscribed dermal-based proliferation with a lobular low-power appearance. At a higher power, well-formed capillary-sized vessels can be identified. The nuclei of the endothelial cells are uniform with significant atypia. These findings are consistent with hemangioma (Figure 18-11).
QUICK REVIEW Rules for Vascular Lesions
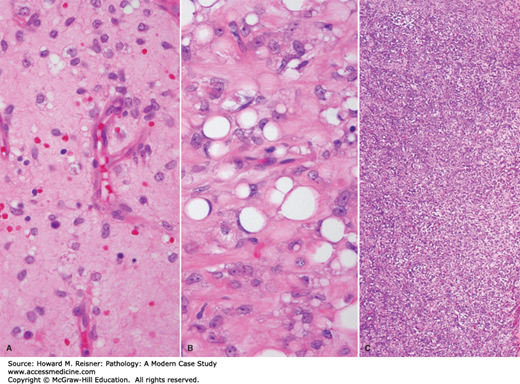
Low-power architecture is critical. Reactive and benign vascular lesions have a well-circumscribed, lobular low-power appearance. Diffuse, infiltrative vascular proliferations are worrisome. Once you have assessed the lesion at low power, features at high power that can be worrisome include nuclear atypia, nuclear stratification, and poorly formed vessels (Figure 18-12) (see Table 18-3).
Vascular differentiation is a spectrum and may be difficult to recognize. Well-differentiated vascular lesions contain recognizable vessels with lumen formation. In some cases, vascular differentiation is in the form of cells with a single intracytoplasmic lumen. Rarely, vascular differentiation is so primitive that the lesion shows no morphologic evidence of endothelial lineage. In this situation, immunohistochemistry is needed to prove vascular differentiation. Helpful immunostains are CD31 and CD34.
| Benign | Malignant | |
|---|---|---|
| Low-power architecture | Circumscribed, lobular | Infiltrative, dissection through collagen |
| Nuclear features | Uniform nuclei | Nuclear atypia and hyperchromasia |
| Nuclear stratification | No | Yes |
| Degree of vascular differentiation | Well-formed vessels | Poorly formed vessels |
FIGURE 18-12
Angiosarcoma. Left (low-power view) compares this diffuse, infiltrative lesion with Figure 18-11 left. Right (high-power view) notes the poorly defined vascular structures. Neoplastic endothelial cells demonstrate hyperchromatic, pleomorphic nuclei that show stratification.
BENIGN VASCULAR TUMORS
Hemangiomas are benign neoplasms of endothelial cells. These lesions are relatively common and occur most frequently in the head and neck region of infants and children. It is often difficult if not impossible to distinguish hemangiomas from arteriovenous malformation. Some regress over time, and others reach a relative stable size. Hemangiomas do not undergo malignant transformation.
Variants: There are several types of hemangioma including lobular capillary hemangioma, pyogenic granuloma, and cherry angioma. While these lesions have varying clinical presentations, they share a common morphologic appearance.
Lobular capillary hemangiomas (see Case 18-2) are characterized by a circumscribed nodular arrangement of small capillary size vessels with a larger feeder-type vessel.
Pyogenic granulomas are polypoid hemangiomas that occur on mucosal surfaces and skin with a granulation tissue appearance. Some are associated with trauma or pregnancy.
Cherry angiomas typically present in adults, usually occur on the trunk or extremities, and have a ruby red appearance.
Some vascular lesions occur in deep soft tissue. Although most of these are benign and do not metastasize, they may recur and even be locally aggressive. Intramuscular hemangioma usually presents as a deep soft tissue mass in the lower extremity of young adults. They many contain small capillary-sized vessels, larger cavernous-sized vessels, or a mix of the two. Some contain a significant amount of adipose tissue and may be mistaken for a lipomatous tumor. Intramuscular hemangiomas may be difficult to excise. Up to 20% recur; they may be challenging to manage clinically.
Papillary endothelial hyperplasia is not a true neoplasm; rather it is a reactive process that usually involves the wall of a preexisting blood vessel or even a vascular neoplasm like a hemangioma. Even though this is a reactive condition, it still follows the rules outlined earlier: circumscribed, bland nuclear features. Complete excision is curative.
CASE 18-3
A 70-year-old man presents with large subcutaneous thigh mass.
Low-power examination reveals a multinodular myxoid neoplasm centered in subcutaneous tissue. The lesional cells are spindled with hyperchromatic atypical nuclei. Arching curvilinear blood vessels are visible. In some areas, the neoplastic cells are more closely packed and exhibit fascicular growth with little intervening myxoid stroma. These features are consistent with myxofibrosarcoma (Figure 18-13).
MALIGNANT VASCULAR TUMORS
Angiosarcoma is a malignant tumor of vascular differentiation. These tumors are uncommon lesions that usually affect adults. The classic clinical presentation is a skin lesion on head/neck of an elderly person. These tumors are very infiltrative (poorly circumscribed) and gross examination often underestimates the true extent of the lesion. For this reason, surgeons may have difficulty completely excising the lesion, and the surgical margins may be positive. Microscopically, these tumors are composed of an intricate network of poorly defined vascular structures diffusely infiltrating the dermis or other tissue. In some cases, primitive vessels may be identified, but in other lesions the neoplastic cells show no evidence of endothelial differentiation (Figure 18-12). The neoplastic cells have hyperchromatic and pleomorphic nuclei and nuclear stratification. Even though mitotic figures should not be used to distinguish between reactive/benign and malignant vascular lesions since reactive/benign lesions can have brisk mitotic activity, the mitotic rate in angiosarcomas is usually high. In cases where the pathologist cannot be confident of endothelial differentiation by H&E morphology, immunohistochemical stains such as CD31 and CD34 can be used. Angiosarcoma is an aggressive tumor, and the overall long-term survival is poor.
Angiosarcoma can occur in several clinical scenarios. Although the most common clinical scenario involves the head/neck of the elderly, angiosarcoma may also arise with chronic lymphedema, following radiation treatment or with exposure to certain chemicals like Thorotrast or vinyl chloride. Despite the clinical differences, the morphologic features are similar, and prognosis is dismal in all types.
FIBROBLASTIC AND MYOFIBROBLASTIC LESIONS
Fibroblasts are slender spindled cells with pale cytoplasm, indistinct cell borders, uniform nuclei, and pinpoint nucleoli. They are responsible for the production of extracellular matrix of fibrous connective tissue including collagen, elastin, and glycosaminoglycans. Myofibroblasts are the most important cell type in tissue repair, and they have overlapping features of fibroblasts and smooth muscle. Microscopically, they are also spindled cells with lightly eosinophilic cytoplasm and bland nuclei, but unlike fibroblasts they contain bundles of intracytoplasmic microfilaments that provide a mechanism of cell contraction.
QUICK REVIEW Rules for Fibroblastic and Myofibroblastic Lesions
Fibroblastic and myofibroblastic lesions can be divided into reactive and neoplastic conditions, and often it is difficult to distinguish the two. Frequently reactive lesions will be more heterogeneous in cellular composition, while fibroblastic/myofibroblastic neoplasms will have a more organized appearance with a regular distribution of blood vessels. While marked cytologic atypia favors a malignant process, some reactive lesions may have some cellular pleomorphism while neoplasms may appear deceptively bland. Similarly, atypical mitotic figures are a worrisome finding, but the mere presence of mitotic activity is generally not helpful diagnostically. Finally, fibroblasts and myofibroblasts variably express smooth muscle markers, but typically immunohistochemical stains are most useful in excluding other entities in the differential diagnosis.
Fibroblastic and myofibroblastic lesions can be characterized as reactive, benign and malignant lesions including nodular fasciitis, fibromatosis, and myxofibrosarcoma.
REACTIVE FIBROBLASTIC LESIONS
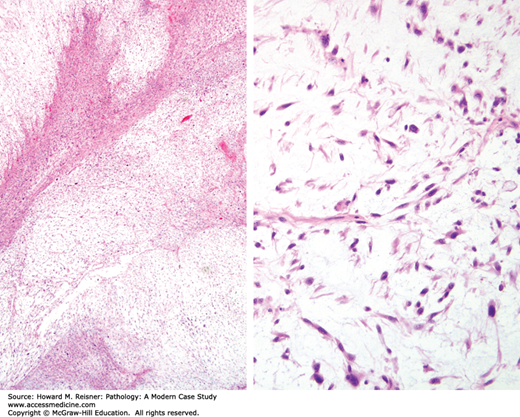
Nodular fasciitis is a self-limited fibroblastic and myofibroblastic proliferation that is often clinically concerning because patients present with a rapidly growing mass. These lesions most commonly affect young adults, in areas such as upper extremity. In children, nodular fasciitis has a predilection for the head and neck. Histologic examination shows bland spindle cells without significant cytologic atypia. Mitotic figures are usually easily found, but no atypical mitotic figures are present (Figure 18-14). These lesions are benign, and they should not recur after excision. Nodular fasciitis is an important entity to be aware of because it is the most common reactive/benign lesion that is misdiagnosed as a sarcoma. Recent work has shown most cases of nodular fasciitis harbor rearrangements of the USP6 gene, supporting the notion that this entity ia a “transient neoplasia”.
BENIGN FIBROBLASTIC TUMORS (FIBROMATOSIS)
Fibromatoses are a group of locally aggressive but nonmetastasizing tumors composed of fibroblasts and myofibroblasts. There are two main types of fibromatosis: superficial and deep. Both superficial and deep groups share are characterized by a bland but infiltrative spindle cell proliferation of fibroblasts and myofibroblasts in long sweeping fascicles with a regular distribution of thin-walled blood vessels. Although these superficial and deep fibromatosis are impossible to distinguish by microscopic examination, they differ in clinical presentation and behavior.
Superficial fibromatosis includes plantar and palmar subtypes. Palmar fibromatosis, also known as Dupuytren contracture, affects up to 20% of people over 65 and presents as a single nodule or multiple small nodules. Plantar fibromatosis also occur in adults but occasionally occur in children and young adults. Although superficial fibromatosis has the same morphologic features as the deep type, they have a much better clinical outcome with surgery reserved for symptomatic cases.
Deep fibromatosis can be divided into abdominal, intra-abdominal, and extra-abdominal subtypes. The abdominal type arises from the musculoaponeurotic structures of the abdominal wall and usually occurs in women of childbearing age. Intra-abdominal fibromatosis includes mesenteric and pelvic fibromatosis. Extra-abdominal fibromatosis mainly affects muscles and overlying fascia of the pelvic and shoulder girdle of young adults. Even though the clinical features of these subtypes are variable, all forms of deep fibromatosis have similar morphologic features. While deep fibromatoses do not metastasize, they can cause significant morbidity and mortality from aggressive local behavior. Recurrence rates following excision vary, but reach almost 80% in some series.
IN TRANSLATION
Almost all deep fibromatosis have mutations in the b-catenin or APC gene causing intranuclear accumulation of β-catenin that results in diffuse staining with β-catenin immunohistochemical stain. Conversely, reactive fibroblastic and myofibroblastic proliferations will be negative for β-catenin. Therefore, immunohistochemistry can be a useful tool in distinguishing fibromatosis from reactive proliferations on small biopsy specimens. Since Gardner syndrome is an autosomal dominant disease caused by germline mutation in the APC gene, affected patients are at a substantially higher risk of deep fibromatosis than the general population. Patients with Gardner syndrome also may present with intestinal polyposis, osteomas, and skin cysts.
MALIGNANT FIBROBLASTIC TUMORS
Myxofibrosarcoma is a malignant fibroblastic lesion that classically presents as a superficial mass in the lower extremity of the elderly. Grossly these tumors are lobular and have a gelatinous cut surface. Microscopically, the amount of myxoid stroma varies by case and correlates with less aggressive course (see Figure 18-13). Conversely, those tumors with increased cellularity are high grade and are at risk of more aggressive behavior including more frequent local recurrence and potential for distant metastasis (see Case 18-13).
FIBROHISTIOCYTIC LESIONS
Fibrohistiocytic lesions are a group of entities that contain a mixture of histiocytes and fibroblasts. Histiocytes are cells derived from monocyte–macrophage lineage. They have eosinophilic cytoplasm containing lysosomes that are enzyme-containing organelles used to break down cellular waste material.
BENIGN FIBROHISTIOCYTIC TUMORS
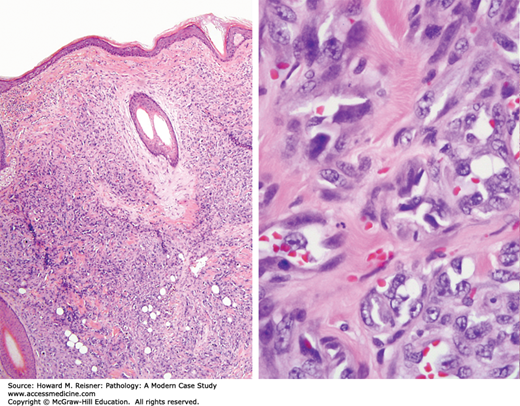
Benign fibrous histiocytoma is a benign lesion that usually present as a solitary slowly growing nodule in adults in the dermis. Microscopically, this lesion consists of a mixed cellular population including spindle cells, xanthoma cells, multinucleated giant cells, inflammatory cells, and hemosiderin-laden macrophages. Epidermal hyperplasia and peripheral collagen trapping are helpful clues to the diagnosis (Figure 18-15). Simple excision is curative as these lesions rarely recur.
Numerous variants of fibrous histiocytoma are recognized and vary in morphology and clinical behavior. For example, atypical fibrous histiocytoma exhibits cytologic atypia and mitotic activity in a background that resembles classic benign fibrous histiocytoma. These variants have a higher recurrence rate than benign fibrous histiocytoma.
QUICK REVIEW Current Trends: Fibrosarcoma And Malignant Fibrous Histiocytoma
It was not long ago that the diagnoses of fibrosarcoma and malignant fibrous histiocytoma (MFH) were two of the most common diagnoses in soft tissue pathology. Fibrosarcoma was described as a malignant spindle cell sarcoma of fibroblasts and typically exhibited a “herringbone” growth pattern. MFH was a pleomorphic sarcoma subdivided into five subtypes: pleomorphic, giant cell, inflammatory, angiomatoid, and myxoid. However, with the advent of improved immunohistochemical and molecular techniques, current thinking about these two entities has changed dramatically. While over 65% of sarcomas were diagnosed as fibrosarcoma in the early 1900s, the diagnosis is virtually extinct. We now realize that many things that were labeled as fibrosarcoma were actually monophasic synovial sarcoma, malignant peripheral nerve sheath tumor (MPNST), and fibrosarcomatous dermatofibrosarcoma protuberans. Similarly, with further investigation we now know that “MFH” is not a reproducible diagnosis, and there is no evidence that tumors with this label are histiocytic in derivation. In fact, many lesions called “MFH” in previous years can now be classified as lymphoma, poorly differentiated carcinoma, dedifferentiated liposarcomas, and various other malignancies. The history of “fibrosarcoma” and “MFH” illustrates the fluidity of the field of pathology and shows how much our understanding of tumors has grown over the past hundred years.
SMOOTH MUSCLE
Smooth muscle is found throughout the body in organs such as the genitourinary tract, gastrointestinal tract, and respiratory tract and provides relatively continuous contractions of low force in a rhythmic or wavelike pattern. The activity of smooth muscle is influenced by the autonomic nervous system, hormones, and local metabolites.
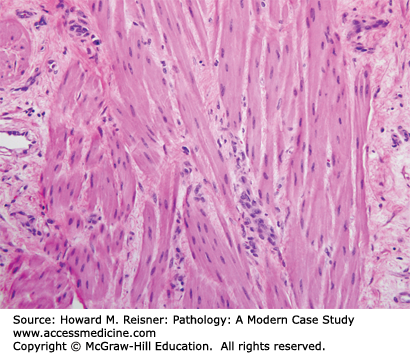
Smooth muscle cells have distinct histologic features and usually can be identified by H&E morphology. One of the characteristic properties of a smooth muscle cell is its brightly eosinophilic cytoplasm. The cells are elongated and spindled, and the nuclei have blunt ends that give them a “cigar-like” appearance. Smooth muscle cells are bound together into fascicles (Figure 18-16). Ultrastructurally, smooth muscle cells have three types of filaments: myosin, actin, and intermediate. Oftentimes when the lineage of spindle cells is in question, immunohistochemistry can help to prove muscle origin by positive staining for actin, desmin, or H-caldesmon.
QUICK REVIEW Rules for Distinguishing Smooth Muscle Neoplasms
Having decided that a tumor is derived from smooth muscle, there are three histologic features to look for: atypia, mitotic figures, and coagulative tumor necrosis. Mitotic figures are the most objective criteria for malignancy, and as a general rule, any mitotic activity in a soft tissue smooth muscle tumor is a worrisome finding. Atypia includes nuclear hyperchromasia or pleomorphism. Atypia is usually subjectively stratified in to mild, moderate, and severe grades. However, any more than mild atypia may be indicative of a malignant tumor. Necrosis in smooth muscle tumors may be of the hyalinized or coagulative-tumor type. In hyalinized-type necrosis, there is a gradual transition between viable and necrotic tissue. In contrast, the demarcation between viable and nonviable tissue is typically sharp in coagulative tumor necrosis and the nuclei in the necrotic cells retain their hematoxyphilia. While hyalinized-type necrosis can be seen in benign smooth muscle neoplasms, coagulative tumor necrosis is a concerning finding.
Superficial or cutaneous leiomyoma can be divided into two types: those arising from pilar arrector muscles and the genital form. Leiomyoma of pilar arrector origin may be solitary or multiple and present as small 1–2 cm brown-red papules that may coalesce. These lesions typically arise during early adulthood and, like angiomyomas, may be painful. Genital leiomyomas are usually solitary and affect sites such as the scrotum, vulva, and areola of the nipple. Microscopically these tumors are composed of bundles of smooth muscle. Genital leiomyomas are usually more circumscribed than the pilar arrector type. Close examination is necessary to make sure that the lesion does not contain worrisome features such as nondegenerative atypia, necrosis, or increased mitotic activity.
Angiomyoma is a benign smooth muscle tumor that classically affects middle-aged women. Patients usually present with a slowly enlarging and painful mass that may be worsened by pressure or change in temperature. Grossly, the mass is circumscribed with a white-gray cut surface. Microscopically, these lesions are composed of smooth muscle concentrically arranged around thick-walled blood vessels. Myxoid change, calcification, and hyalinization may be seen. While degenerative atypia may be seen, these lesions should not have mitotic activity or necrosis. Simple excision is curative.
CASE 18-4
A 65-year-old woman presents with a large retroperitoneal mass.
Histologic sections show an atypical spindle cell proliferation composed of cells with brightly eosinophilic cytoplasm and organized in a fascicular growth pattern. These morphologic features are suggestive of smooth muscle origin. Smooth muscle actin and desmin immunohistochemical stains are strongly positive in the neoplastic population. Examination at higher power reveals significant cytologic atypia and frequent mitotic figures. These findings are consistent with leiomyosarcoma (Figure 18-17).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree