Soft capsules
Keith G. Hutchison and Josephine Ferdinando
Chapter contents
Description of the soft gelatin capsule dosage form (softgels)
Rationale for the selection of softgels as a dosage form
Improved drug absorption characteristics
Patient compliance and consumer preference
Safety for potent and cytotoxic drugs
Oils and low melting point drugs
Dose uniformity of low-dose drugs
Properties of soft gelatin shells
Formulation of softgel fill materials
Product quality considerations
Key points
• They can be used as a formulation approach with the potential to:
• improve patient compliance and consumer preference
• improve manufacturing safety for potent and cytotoxic drugs
• improve manufacturability of low melting point and low dose drugs.
Introduction
When pharmaceutical formulation scientists are designing a solid oral dosage form for drug compounds, they have a number of choices which can be influenced by consumer preference/compliance, economics and technical feasibility. Over recent years, new drug molecules tend to be less soluble in aqueous systems and if intended for oral administration, this can present a considerable formulation challenge for delivering drug for absorption at the desired rate and extent. One approach is to make a liquid formulation containing the drug either in solution or suspended in a matrix more readily dissolved on contact with gastrointestinal media. In order to convert a liquid formula into a solid dosage form, it may be encapsulated into soft gelatin capsules, also known as softgels.
This chapter explains:
Description of the soft gelatin capsule dosage form (softgels)
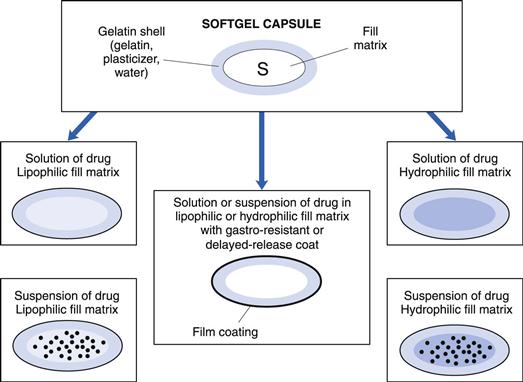
Softgels consist of a liquid or a semi-solid matrix inside a one-piece outer gelatin shell (Fig. 34.1). Ingredients that are solid at room temperature can also be encapsulated into softgels providing they are at least semi-solid below approximately 40 °C. The drug compound itself may be either in solution or in suspension in the capsule-fill matrix. The characteristics of the fill matrix may be hydrophilic (for example, polyethylene glycols), lipophilic (such as triglyceride vegetable oils), or a combination of hydrophilic and lipophilic ingredients (see also Fig. 34.1).
Significant advances have been made in recent years regarding the formulation of softgel fill matrices (Gullapalli 2010). These include self-emulsifying micro-emulsions and nano-emulsions encapsulated as preconcentrates in softgels. The term ‘preconcentrate’ means that the softgel fill matrix which is a combination of lipophilic and hydrophilic liquids as well as surfactant components disperses after oral administration to form an emulsion, with a droplet size either in the micro- or nanometre size range.
The softgel capsule shell consists of gelatin, water and a plasticizer. The shell may be transparent or opaque and can be coloured and flavoured if desired. Preservatives are not normally required owing to the low water activity in the finished product. The softgel can be coated with enteric-resistant or delayed-release coating materials. Although virtually any shape of softgel can be made, oval or oblong shapes are usually selected for oral administration.
Softgels can be formulated and manufactured to produce a number of different drug delivery systems:
• Orally administered softgels containing solutions or suspensions that release their contents in the stomach in an easy-to-swallow, convenient unit dose form (Fig. 34.2)
• Twist-off softgels, which are designed with a tag to be twisted or snipped off, thereby allowing access to the fill material. This type of softgel can be used for unit dosing of topical medication, inhalations or for oral dosing of a paediatric product (Fig. 34.3)
• Meltable softgels designed for use as pessaries or suppositories.
Rationale for the selection of softgels as a dosage form
Some of the reasons why softgels may be selected as the preferred formulation approach are summarized in Table 34.1 and a more detailed description follows. Whilst softgels can solve various technical formulation challenges not possible with tablets, consideration should be given to the fact that they can be more costly than tablet formulations and require specialized manufacturing equipment.
Table 34.1
Summary of the key features and advantages of the softgel dose form
| Features | Advantages |
| Improved drug absorption | Improved rate and extent of absorption and/or reduced variability, mainly for poorly water-soluble drugs |
| Patient compliance and consumer preference | Easy to swallow. Absence of poor taste or other sensory problem. Convenient administration of a liquid-drug dosage form |
| Safety – potent and cytotoxic drugs | Avoids dust-handling problems during dosage form manufacture; better operator safety and environmental controls |
| Oils and low melting point drugs | Overcomes problems with manufacture as compressed tablet or hard-shell capsules |
| Dose uniformity for low-dose drugs | Liquid flow during dosage form manufacture is more precise than powder flow. Drug solutions provide better homogeneity than powder or granule mixtures |
| Product stability | Drugs are protected against oxidative degradation by lipid vehicles and softgel capsule shells |
Improved drug absorption characteristics
Increased rate of absorption
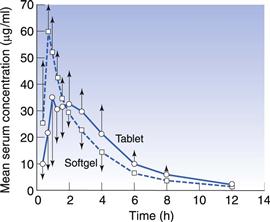
Major advances have been made in the area of developing softgel formulations to address drug absorption issues (Ferdinando 2000, Perlman et al 2008, Aboul-Einien 2009). For poorly water-soluble drugs, ideally the dosage form would present the drug to the gastrointestinal tract in solution form, from which the drug can be rapidly absorbed. This can be achieved using a drug-solution matrix in a softgel formulation and such formulations can provide faster absorption than from other solid oral dosage forms, such as compressed tablets (Lissy et al 2010). This is probably because absorption of a poorly soluble drug from a tablet formulation requires time for disintegration of the tablet into granules, then drug dissolution into gastrointestinal fluid. With the solution-softgel approach, the shell ruptures within minutes to release the drug solution, which can be in a hydrophilic or highly dispersing vehicle that aids the rate of drug absorption. This may be beneficial for (a) therapeutic reasons, such as the treatment of migraine or acute pain, or (b) where there is a limited absorptive region or ‘absorption window’ high in the gastrointestinal tract. Figure 34.4 shows the faster absorption that can be achieved using a solution-softgel formulation of ibuprofen compared to a tablet (Saano et al 1991).
Increased bioavailability
As well as increasing the rate of absorption, softgels may improve the extent of absorption (Aboul-Einien 2009). This can be particularly effective for drugs with poor aqueous solubility and a relatively high molecular weight. An example of such a product is the protease inhibitor saquinavir, which was formulated as a solution-softgel product (Perry & Noble 1988). The solution-softgel formulation provided around three times greater bioavailability than a saquinavir hard-shell capsule formulation as measured by the area under the plasma – time curve (AUC).
In some cases a drug may be solubilized in vehicles that are capable of spontaneously dispersing into an emulsion on contact with gastrointestinal fluid. This is known as a self-emulsifying drug delivery system (SEDDS) (Gao et al 2006). Drug may be dissolved in an oil/surfactant vehicle that produces a micro-emulsion or a nano-emulsion on contact with gastrointestinal fluids. A nano-emulsion of progesterone has been developed whereby the vehicle consists of oils and surfactants in appropriate proportions. On contact with aqueous fluids, it produces an emulsion with an average droplet size less than 100 nm. The solubility of the drug is maintained as long as possible, delivering solubilized drug directly to the enterocyte membrane. This can increase bioavailability compared to formulations in which the drug is dosed in the solid state. Figure 34.5 shows the plasma concentration – time profile for progesterone absorbed from the nano-emulsion formulation (Ferdinando 2000).
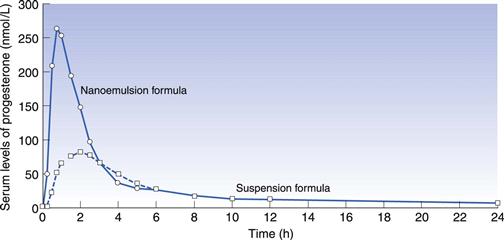
Fig. 34.5 Pharmacokinetic evaluation of progesterone comparing a softgel nano-emulsion solution of progesterone with a softgel containing a suspension of the drug in an oil following single dose administration in 12 healthy human volunteers. Reproduced from Ferdinando 2000.
Softgel formulations may contain excipients, for example one or more surfactants that can aid stability, wettability or even enhance permeability of the drug (Aungst 2000).
Decreased plasma variability
High variability in drug plasma levels is a common characteristic of drugs with limited bioavailability. By dosing drug optimally in solution, the plasma level variability of such drugs can be significantly reduced, particularly if absorption is limited by drug solubility. SEDDS have been shown to reduce variability of exposure to the lipophilic drug torcetrapib compared to a formulation in oil (Perlman et al 2008). The cyclic polypeptide drug ciclosporin (Sandimmune Neoral®) benefits from such an approach by using a micro-emulsion preconcentrate in a softgel (Drewe et al 1992, Meinzer 1993).
Patient compliance and consumer preference
A number of self-medicating consumer preference studies have been carried out to gauge the user’s perception of softgels relative to hard-shell capsules and tablets. The results of the studies showed that consumers expressed their preference for softgels in terms of (a) ease of swallowing, (b) absence of taste and (c) convenience in use.
This expressed appeal of the softgel dosage form may have a positive impact on patient compliance. Compliance may be further enhanced if the softgel formulation enables dosing of smaller or fewer dosage units, as a result of increased bioavailability.
Safety for potent and cytotoxic drugs
The mixing, granulation and compression/filling processes used in preparing tablets and hard-shell capsules can generate a significant quantity of airborne powders. This can be a cause of concern for the manufacture of highly potent or cytotoxic compounds because of safety considerations for the operator and environment.
By preparing a solution or suspension of drug, where the active component is essentially protected from the environment by the liquid, these safety concerns can be reduced.
Oils and low melting point drugs
When the pharmaceutical active is an oily liquid, has a melting point lower than about 75 °C or proves difficult to compress, liquid filling of softgels (with or without other diluents) can provide a successful approach to presenting it in a solid oral dosage form.
Dose uniformity of low-dose drugs
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree