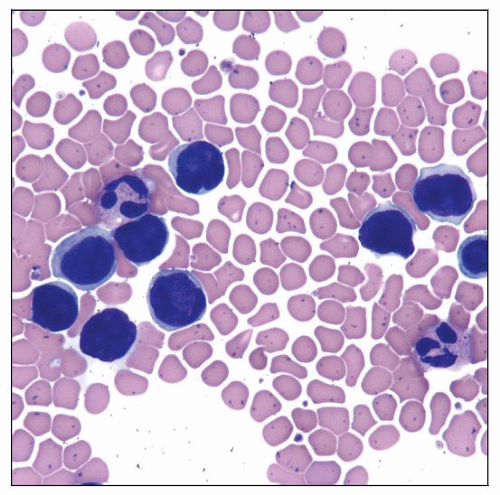
Sezary Syndrome
Sa A. Wang, MD
Key Facts
Terminology
Sézary syndrome (SS) is defined by triad of
Pruritic erythroderma
High number of Sézary cells in blood (> 1,000/µL )
Lymphadenopathy, usually generalized
Clinical Issues
SS presents de novo in most patients
Can be preceded by mycosis fungoides (MF)
Cases should be designated as “SS preceded by MF”
Skin: Intractable pruritus and generalized erythroderma with edema (≥ 80% of skin surface)
Extracutaneous sites of involvement
Lymph nodes, lung, liver
Spleen, central nervous system, other organs
Bone marrow is relatively spared
Most treatments are palliative, not curative
Total skin electron beam radiation with nonmyeloablative allogeneic SCT may be curative
Poor survival: Median < 2.5 years
Microscopic Pathology
Sézary cells (cells with cerebriform nuclei) in peripheral blood
Skin changes are very similar to mycosis fungoides
Lymph nodes show partial or total effacement of normal architecture
Top Differential Diagnoses
Nonneoplastic causes of erythroderma
Adult T-cell leukemia/lymphoma (ATLL)
T-cell prolymphocytic leukemia
Leukemia cutis (especially monocytic leukemia)
Peripheral T-cell lymphoma, not otherwise specified
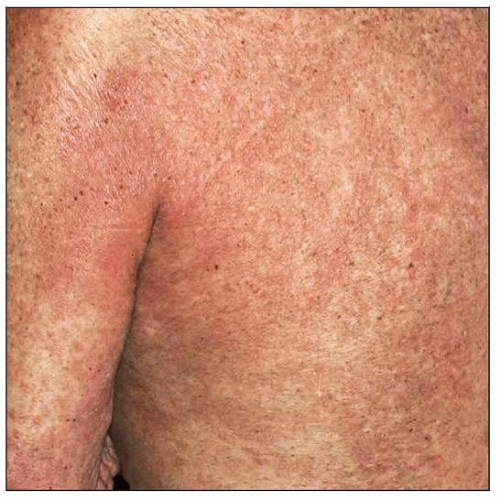 Skin of patient with Sézary syndrome shows erythroderma. The patient’s back and left arm are shown in this field. |
TERMINOLOGY
Abbreviations
Sézary syndrome (SS)
Synonyms
Erythrodermic cutaneous T-cell lymphoma (E-CTCL)
Definitions
SS defined by triad of
Pruritic erythroderma
High number of Sézary cells in peripheral blood (> 1,000/µL ) with
Confirmed T-cell clonality or
Increased CD4:CD8 ratio > 10 or
T-cell immunophenotypic aberrancies demonstrated by flow cytometric immunophenotyping
Lymphadenopathy, usually generalized
ETIOLOGY/PATHOGENESIS
Unknown
Genetics, infectious agents, or environmental exposures may play a role
CLINICAL ISSUES
Epidemiology
Incidence
5% of all cutaneous T-cell lymphomas
Age
Adults; median: 60 years (range: 45-70 years)
Gender
M:F = 1.5:1
Ethnicity
Incidence in blacks 2x as high as in whites
Presentation
SS occurs de novo in most patients
Can be preceded by prodromal phase
Pruritus or nonspecific dermatitis
Can be preceded by mycosis fungoides (MF)
Patients must fulfill blood criteria for SS (T4B2)
These cases should be designated as “SS preceded by MF”
Recommendation of International Society for Cutaneous Lymphomas (ISCL)
Skin: Intractable pruritus and generalized erythroderma with edema (≥ 80% of skin surface)
Associated with alopecia, ectropion, leonine facies
Nail dystrophy, plantar hyperkeratoses with extremely painful fissuring
Secondary bacterial infection
Some cases show marked photosensitivity
Mimic chronic actinic dermatitis
Extracutaneous involvement
Lymph nodes
Liver, lung, spleen, central nervous system, and any other organs
Bone marrow is relatively spared
Increased prevalence of secondary malignancies, especially lymphoma
May be attributable to decreased normal CD4(+) T cells
Hypereosinophilic syndrome has been reported to be associated with SS
Can cause end organ dysfunction
Laboratory Tests
Work-up should include
Complete blood count with differential
Serum chemistry tests of liver and renal function, electrolytes, and lactate dehydrogenase (LDH)
Serologic tests for viruses
HTLV-1, HIV, hepatitis B
Flow cytometric immunophenotyping of peripheral blood useful for
Confirming clonality
Detecting immunophenotypic aberrancies
Molecular analysis of T-cell receptor (TCR) genes for assessment of clonality
T-cell clone can be seen in up to 20% of reactive skin conditions
Treatment
Most therapies are palliative and not curative
Extracorporeal photoimmunotherapy
Bexarotene (retinoid)
Methotrexate
Vorinostat (histone deacetylase inhibitor)
Alemtuzumab (anti-CD52)
Denileukin diftitox (anti-CD25 IL-2 diphtheria fusion protein)
High-dose chemotherapy
Etoposide, vincristine, doxorubicin, bolus cyclophosphamide, oral prednisone (EPOCH)
Autologous hematopoietic stem-cell transplantation following high-dose chemotherapy
Can produce remissions, but early relapses are common
Total skin electron beam radiation with nonmyeloablative allogeneic stem cell transplantation (SCT)
Possibly curative approach
Prognosis
Poor; median survival < 2.5 years
Predictors of poor prognosis
Advanced age
Elevated serum LDH
MICROSCOPIC PATHOLOGY
Histologic Features
Peripheral blood
Cells with cerebriform nuclei (Sézary cells)
Can range in size from small to large
Small Sézary cells (< 12 µm in diameter)
Large Sézary cells (> 14 µm in diameter)
Sézary cells are not completely specific
Especially small forms can be seen in reactive conditions
Most large cells are neoplastic
Skin
Changes are very similar to MF
In ˜ 2/3 patients with SS, skin biopsy shows diagnostic findings
Epidermotropism is variable
Can be absent in some biopsy specimens
Atypical cells are present only in dermis; often perivascular
Tumor cell size can be variable
Cell population is often monotonous (more so than MF)
In ˜ 1/3 of patients, only nonspecific changes without abnormal lymphocytes
Cannot distinguish from nonneoplastic erythroderma using histologic criteria
Bone marrow
Often not involved or only minimal involvement
When involved, Sézary cell infiltrate is often sparse
Mainly interstitial pattern; often patchy
Cytologic Features
Cerebriform cells; can be small, medium, or large
Lymph Nodes
Involved lymph nodes show partial or total effacement of normal architecture by Sézary cells
Dense, monotonous infiltrate of Sézary cells
Capsular invasion or extranodal invasion is often present
B-cell follicles may be reduced in number or small
Changes of dermatopathic lymphadenopathy are often present with
ANCILLARY TESTS
Immunohistochemistry
Pan-T-cell antigens(+), TCR-αβ(+)
Loss or dim expression of T-cell antigens(+/-)
CD4(+), CD8(-)
CD25(-/+), CD30(-/+), CD52(+)
B-cell antigens(-)
Ki-67 moderate to high
Flow Cytometry
CD2(+), CD3(+), CD5(+), CD7(+), TCR-αβ(+)
CD4(+), CD8(-)
TdT(-), CD1a(-), CD10(-), B-cell antigens(-)
Immunophenotypic aberrancies are common; best detected by flow cytometry
Increased CD4:CD8 ratio
Loss of CD7, CD26, or other antigens
Altered expression levels of CD2, CD3, CD4, or CD5
Vβ analysis is useful to confirm clonality and for quantifying neoplastic cells
Can be used for initial diagnosis and monitoring treatment response
Cytogenetics
No specific chromosomal abnormalities
Complex karyotypes are common
Both numeric and structural abnormalities are observed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree