Selective Vagotomy and Pyloroplasty
Steven D. Schwaitzberg
Robert W. Bailey
Jose M. Martinez
Keith A. Kelly
Sumeet S. Teotia
Introduction
Proximal gastric vagotomy (PGV), also called highly selective vagotomy (HSV) or parietal cell vagotomy, was first introduced in the 1950s as a definitive treatment for chronic peptic ulcer disease. When compared to other open procedures like vagotomy/antrectomy or vagotomy/pyloroplasty, an HSV has been shown to have a decreased morbidity in terms of motility complications or risk of anastomotic leak and a negligible mortality. When medical treatment fails, it remains as one of the preferred operations for the elective surgical treatment of chronic duodenal ulcer as well as selective acute indications. This operation selectively in-terrupts the vagal branches to the acid-producing (parietal) cells in the fundus and corpus of the stomach, but leaves intact the nerves of Latarjet, the celiac and the hepatic vagal branches. By preserving the vagal branches to the antrum and pylorus, gastric emptying is maintained, thus avoiding the need for a gastric drainage procedure. Acid secretion is significantly reduced. Healing of chronic duodenal ulcers occurs in approximately 70% to 80% of patients. Additional advantages of this operation are that it is safe and the side effects (e.g., gastric stasis, bile reflux, the dumping syndrome, and diarrhea) that commonly follow truncal vagotomy and “drainage operations” or gastrectomy seldom occur after HSV. Although the technical aspects of HSV are more demanding than they are with truncal vagotomy, once mastered and when performed correctly, the advantages of HSV outweigh the disadvantages in appropriately selected patients. Today on almost every front, laparoscopic antiulcer surgery has evolved to imitate its traditional open counterpart. Initially, laparoscopic variations to a standard HSV were introduced. Most of these procedures involved a posterior truncal vagotomy combined with an anterior seromyotomy, an anterior linear gastrectomy, or an anterior HSV. As video systems, tools, and techniques have evolved, these techniques have now been abandoned in favor of the more traditional approach to the technique. In many centers, a laparoscopic HSV has become the procedure of choice for the management of chronic duodenal ulcers refractory to medical management
Due to its attractive benefits, HSV gained popularity in the 1960s, 1970s, and 1980s. Today, the incidence of peptic ulcer disease requiring surgical intervention via any approach has decreased dramatically over the last 20 years due to the understanding of the Helicobacter pylori infection and the ubiquity of proton pump inhibitors. In this chapter, we outline the current indications for this procedure and discuss both the open and laparoscopic approaches.
HSV is indicated in patients who have chronic duodenal ulcer and in whom adequate medical treatment for H. pylori infection has been unsuccessful in eliminating the ulcer. Sometimes, it is also the procedure of choice in stable patients who have a perforated or bleeding duodenal ulcer. It has been established that a significant percentage of patients who present acutely in this fashion are actually not infected with H. pylori, which adds to the attractiveness of an anti-acid procedure in this setting. The perforated ulcer can be closed with an omental patch, or the bleeding ulcer can be suture-legated via a duodenotomy, after which the HSV can be performed. This type of vagotomy is also an excellent choice for patients who present with gastric outlet obstruction from a stenosing duodenal ulcer. In contrast to the outcome when truncal vagotomy is used in this setting, only <5% of patients who receive HSV and pyloroplasty for this condition have prolonged postoperative gastric stasis. In contrast, <30% of patients who have truncal vagotomy and pyloroplasty for this condition have such stasis.
Preoperative Evaluation/Patient Selection
The preoperative evaluation and indications for open or laparoscopic HSV are similar. Besides routine preoperative blood testing, serum gastrin levels should be obtained to rule out Zollinger–Ellison syndrome. All patients should undergo preoperative upper gastrointestinal endoscopy within 12 months of their scheduled surgery. Appropriate
biopsies are obtained to prove eradication of H. pylori. Alternatively, a stool specimen to rule out H. pylori can be obtained to rule out active infestation. Serum studies are less useful since antibodies do not revert to undetectable once the infection is cleared. Preoperative upper endoscopy is required to rule out bleeding or nonhealing gastric ulcers since HSV is indicated for these conditions.
biopsies are obtained to prove eradication of H. pylori. Alternatively, a stool specimen to rule out H. pylori can be obtained to rule out active infestation. Serum studies are less useful since antibodies do not revert to undetectable once the infection is cleared. Preoperative upper endoscopy is required to rule out bleeding or nonhealing gastric ulcers since HSV is indicated for these conditions.
The most common indication for surgery is for refractory duodenal ulcer disease and its associated complications of obstruction, bleeding, or perforation. Laparoscopic or open HSV for complicated duodenal ulcer disease should be limited to hemodynamically stable patients and performed by surgeons with adequate experience.
After induction of general anesthesia, a nasogastric tube and Foley catheter should be inserted. Preoperative antibiotics are usually unnecessary for patients undergoing HSV. However, in patients who have bleeding or perforated duodenal ulcers and who have an obstructing duodenal ulcer when a pyloroplasty is also contemplated, a first-generation cephalosporin, and should be given before and during the operation to decrease the risk of postoperative intra-abdominal and wound infection. In the event of gastric obstruction, some consideration to oral antibiotics should be made as well. Pneumatic compression boots are applied to prevent deep venous thrombosis (DVT) in the legs during the operation. Additional pharmacologic therapy should be considered in patients with an elevated risk profile for DVT.
Operation: Open Highly Selective Vagotomy
Positioning, Anesthesia, and Skin Preparation
The patient is placed supine on the operating table. The arms can be tucked along the side of the body or abducted 90 degrees from the body. The operating table is tilted slightly head-up in reverse Trendelenburg position to allow the abdominal viscera to be drawn downward by gravitational forces. General endotracheal anesthesia with muscle relaxation is used. The patient’s lower chest and abdomen are prepared with an antiseptic solution from the nipple line to the pubis. The upper drape should be placed well above the xiphoid.
Incision and Exposure
An upper abdominal midline incision is made from the xiphoid at the superior aspect, skirting around the umbilicus to the left and ending just below the umbilicus. It is often helpful to carry the incision in the fascia up and along the xiphoid cartilage gaining as much as 2 cm of cephalad exposure. The peritoneal cavity is entered and the round ligament divided. A Buckwalter or similar self-retaining retractor is inserted to elevate the sternum and anterior costal margin and to spread the wound. The liver is retracted anteriorly and to the right with a flat, broad abdominal retractor to expose the stomach, the duodenum, and the esophagogastric junction. Division of the left triangular ligament of the liver is not usually necessary at this point to accomplish good exposure.
Exploration and Ulcer Management
The abdomen is carefully explored to confirm the diagnosis of chronic duodenal ulcer and to rule out other morbid conditions. Perforated duodenal ulcers should be managed by an omental (Graham) patch technique. Bleeding ulcers should be sutured transluminally via an anterior longitudinal duodenotomy closed longitudinally. Stenosing ulcers at the pylorus or duodenum should be ruled out intra/preoperatively by endoscopy; however, when this is not possible, a 28-French orogastric tube can be manipulated through the pylorus and into the distal second portion of the duodenum. An inflammatory narrowing can usually be easily dilated with the tube or the index finger passed through a 2-cm anterior antrotomy. A fibrous stenosis should be managed by a single-layer full-thickness Heineke–Mikulicz pyloroplasty constructed with 3-0 silk. This should be <7 cm in length and centered at the pylorus. Nonperforated, nonobstructing, and nonbleeding ulcers need no operative management. Care must be taken not to disturb the lesser curve of the stomach and the gastrohepatic omentum is important since manipulation in this region may result in edema and make clear identification of the nerves of Latarjet more difficult.
Vagotomy
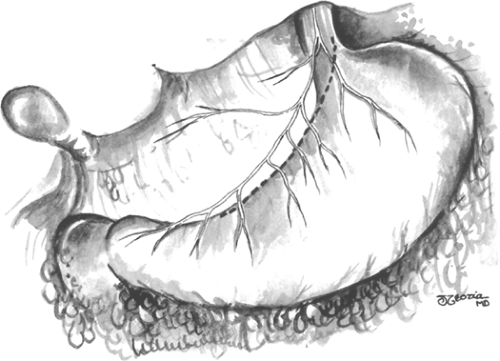
The pylorus is identified by recognizing the Veins of Mayo and pyloric muscle. A point on the lesser curvature of the stomach 7 cm proximal to the pylorus is marked. This is the point at which the vagotomy starts. The vagal branches to the lesser curve are visible. They run in two major leaflets (anterior and posterior) and these layers should be divided separately to avoid encroaching on the posterior nerve of Latarjet. The vagal branches to the distal 7 cm of the stomach, the antrum, and the pylorus are spared (Fig. 1). The first assistant places the stomach on traction by pulling inferiorly on the stomach, using the nasogastric tube as a handle.
Alternatively, a window in the gastrocolic ligament along the greater curve can be fashioned to serve as a handle. Care must be taken to identify and divide any anterior attachments of the greater omentum to the spleen before this step is performed to minimize the risk injury.
Alternatively, a window in the gastrocolic ligament along the greater curve can be fashioned to serve as a handle. Care must be taken to identify and divide any anterior attachments of the greater omentum to the spleen before this step is performed to minimize the risk injury.
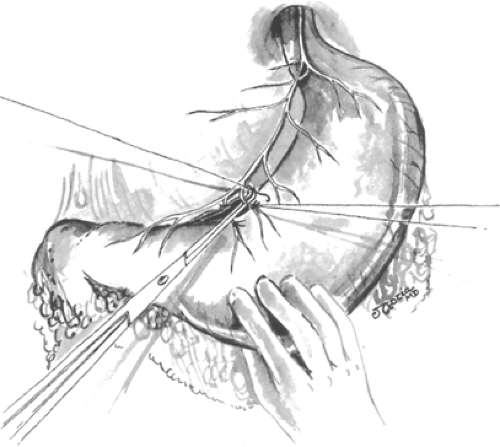
The distal most branch of the anterior nerve of Latarjet to be divided is identified and ligated. The nerves of Latarjet course in the lesser omentum <1 cm lateral to the lesser curvature and parallel to it. Starting from the previously marked point on the stomach 7 cm proximal to the pylorus and working toward the esophagus, the blood vessels and nerves at the junction of the lesser omentum and the anterior gastric wall are divided and ligated with sutures, or ligated in continuity and divided (Fig. 2). An ultrasonic dissector or radiofrequency cautery devices may also be used to divide these vessels and nerves if care is used to avoid thermal injury to the partially devascularized lesser curve of the stomach. Despite these concerns, it is imperative that these structures be divided close to the wall of the stomach to ensure preservation of the nerves of Latarjet. This dissection continues along the lesser curvature up to the esophagogastric junction for a few centimeters.
Dissection of the Esophagogastric Junction and Esophagus
At this point, it may be necessary to divide the triangular ligament to the left lobe of the liver and fold the left lateral segment downward and to the right. The esophagogastric junction is freed from its attachments to the diaphragm, crura, and retroperitoneum. The dissection is adequate only when the entire junction can be completely lifted anteriorly from its usual position demonstrating a circumferential dissection. The anterior vagal nerve trunks can be identified by feeling of the “bow string” along the left anterior wall of the esophagus. Leftward traction on the esophagus will often reveal the posterior vagal trunk just anterior to the aorta if not running along the posterior wall of the esophagus. To avoid injuring the vagal trunks at this point, all dissection around the esophagus should be performed close to the esophageal wall. By lifting the anterior vagus nerve anteriorly, the plane between the nerve and the stomach is continued until the dissection reaches the portion of the vagotomy initiated along the lesser curve. These maneuvers are repeated along the posterior leaf, where the dissection proceeds once again toward the hiatus for a convenient distance. The posterior vagus nerve trunk is then elevated and retracted toward the right allowing the posterior leaflet of the gastrohepatic omentum to be separated from the stomach until the previous dissection is reached (Fig. 3).
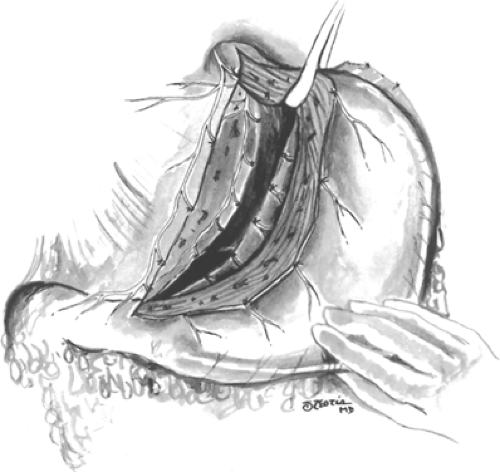 Fig. 3. With the stomach held down by the first assistant grasping it around the nasogastric tube, the remainder of the dissection is performed from the front. When the esophagogastric junction is reached, a soft rubber drain is passed around the esophagus so that it can be held up and to the left. This step makes the division of the last few strands of tissue passing to the right-hand side of the esophagus easier. It is essential to lay bare at least 5 cm of the esophageal muscle and to visualize the posterior vagus as it enters the abdomen between the crura during this part of the dissection.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|