Selective Vagotomy and Pyloroplasty
Steven D. Schwaitzberg
John L. Sawyers
William O. Richards
Surgical treatment of peptic ulcer disease is uncommon today, but was once one of the most frequent types of operations performed by general surgeons. In the heyday of the surgery for peptic ulcer disease, every surgeon and trainee knew the litany of procedures and the commonly accepted recurrence rates and complications for the major ulcer operations. Today, it is uncommon to see cases posted for nonemergent treatment of ulcer disease, although emergencies do occur from time to time. Surgeons trained in the last 15 years have had little opportunity to hone the skills needed to perform safe and accurate vagotomy of the major trunks, not to mention the somewhat more elegant dissection of the nerves of Latarjet needed to perform a highly selective vagotomy. Thus, what is to become of the middle child, “selective vagotomy”? With surgeons emerging from training today with little or no experience in the classic surgical management of peptic ulcer disease, those who are confronted by the occasional perforated or bleeding duodenal ulcer will often choose the most straightforward approaches available to them.
Selective vagotomy was conceived in order to affect complete vagal denervation of the stomach, yet at the same time preserve vagal innervation of the hepatobiliary and celiac branches. Common criticism of selective vagotomy is that it is needlessly complex. Recent review of the literature concerning selective vagotomy reveals almost no journal contributions in several years. Perhaps it is only the foregut aficionado who will retain the knowledge and interest in order to perform this procedure, although there will be situations in which this might be the preferred procedure. Certainly in the case of acute perforation in a patient who is Helicobacter pylori-negative, inflammation may obliterate the view of the nerves of Latarjet, making highly selective vagotomy problematic; thus, it is critical to retain the knowledge of all of the options available for the treatment of this disease.
Selective vagotomy was developed more than 50 years ago as a refinement of bilateral truncal vagotomy. Specifically, the anterior vagal parasympathetic branches to the hepatobiliary structures and those innervated by the celiac branch of the posterior vagus nerve are preserved. At the same time, the parasympathetic innervation to the entire stomach is divided. In theory, this will result in significant decrease in acid reduction while preserving the innervation to the pancreas biliary tree and intestines. The additional complexity of this procedure was justified by an apparent reduction in the incidence of diarrhea noted after couple vagotomy. Diarrhea was reported in as many as 70% of patients in whom truncal vagotomy was performed and was noted to be lessened in those patients who underwent selective vagotomy. The benefits of preserving the anterior vagal hepatobiliary branches are more controversial. There is conflicting evidence of the effect of truncal vagotomy on gallbladder motility, with the net decrease in bile flow possibly resulting from vagally mediated increased resistance to bile flow through the sphincter of Oddi.
A prospective randomized clinical study comparing the effects of truncal and selective vagotomy in 143 patients is summarized in Table 1. There was no statistically significant difference between the two groups of patients in regard to nutrition, weight loss, anemia, acidity, or dumping syndrome. Diarrhea was only slightly more common in those patients undergoing truncal vagotomy, but this difference was not statistically significant. The only important difference between the two groups in this study was the completeness of the vagotomy to the gastric mass as measured by the Hollander test. In order to determine the completeness of vagotomy, insulin is administered to the patient. If the vagotomy is complete, the acid production falls relative to the preinsulin values (negative test). In the event of persistent vagal innervation, acid secretion will increase in response to insulin administration (positive test). Postoperative Hollander tests were positive in 19% of patients after truncal vagotomy and in only 2% of patients after selective vagotomy. This may be caused by a somewhat more thorough dissection around the esophagus.
Table 1 Evaluation of Truncal versus Selective Vagotomy | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Prior to the understanding that most patients with peptic ulcer disease could be treated by eradicating their H. pylori infection, it was highly selective vagotomy that had emerged as the preferred operation by many surgeons for the operative treatment of chronic ulcer disease. The fact that the preservation of the nerves of Latarjet is more technically demanding is offset by the fact that most patients do not require a drainage procedure because the innervation to the antrum is preserved. The choice of operative procedure in this day and age has never been more complex, given the fact that there are almost no large series comparing procedures in the last decade. Reviews of large series, such as those presented in the early 1990s that span nearly a quarter of a century of follow-up, simply do not appear today in which all patients with ulcer-related infection would be treated. Despite this, a few observations can be gleaned from these prior studies. The ulcer recurrence rate after proximal gastric vagotomy has been consistently higher in most reported series than that after selective gastric vagotomy with pyloroplasty. For example, the Arhus County Vagotomy Trial reported an ulcer recurrence rate for patients with duodenal ulcer treated by proximal gastric vagotomy of 15%, compared with 9% after selective gastric vagotomy and a drainage procedure. Griffith, in a 12- to 17-year follow-up study of patients undergoing selective vagotomy plus pyloroplasty, reported 5 ulcerations occurring in 87 patients: 1 stomal ulcer, 2 gastric ulcers, and 2 instances of hemorrhagic
gastritis. In his earlier report, Griffith reported only 1 ulcer recurrence in 103 patients followed for 4 to 9 years after selective vagotomy and pyloroplasty for duodenal ulcer. Three of these gastric ulcers were thought to be secondary to biliary reflux because the insulin test for completeness of vagotomy was negative in these patients.
gastritis. In his earlier report, Griffith reported only 1 ulcer recurrence in 103 patients followed for 4 to 9 years after selective vagotomy and pyloroplasty for duodenal ulcer. Three of these gastric ulcers were thought to be secondary to biliary reflux because the insulin test for completeness of vagotomy was negative in these patients.
Studies from various groups in Copenhagen report different ulcer recurrence rates after selective vagotomy with pyloroplasty. After 5 to 8 years of a prospective, randomized follow-up study, Madsen and Kronborg reported an ulcer recurrence rate of 14% after selective vagotomy and pyloroplasty. The ulcer recurrence rate after proximal gastric vagotomy was 26%, almost twice as high. In 1994, a group from Copenhagen reported a 23-year study of patients undergoing parietal cell (selective proximal) vagotomy in 347 patients with a recurrent ulcer rate of 21.9%. These investigators concluded that the rate of recurrent ulceration after parietal cell vagotomy is proportional to the duration of follow-up. Almost 80% of patients with recurrent ulcer developed their recurrence 10 years or more after their operations. This increasing incidence of ulcer recurrence with length of follow-up has not been reported after selective vagotomy and pyloroplasty.
Choice of Drainage Procedures with Selective Vagotomy
It is commonly held that denervation of the gastric mass by truncal or selective vagotomy will result in gastric stasis; therefore, a drainage procedure aimed at providing an adequate gastric outlet is performed with the vagotomy. Notwithstanding, there is some recent evidence that this effect can be transitory and could possibly be managed with pyloric dilatation and prokinetic motility agents, with drainage required in only a small number of patients. The two classic drainage procedures are pyloroplasty and antrectomy. When associated with truncal vagotomy the incidence of recurrence of ulcer disease is ∼2% when performed in conjunction with antrectomy. This recurrence rate can rise as much as 10-fold when truncal vagotomy is combined with pyloroplasty. The same difference was not observed in a prospective randomized trial comparing drainage procedures used in conjunction with selective vagotomy. Accounting for these observations is difficult; however, a few inferences can be made. The use of antrectomy likely affords protection against ulcer recurrence through the reduction of the antral mass and ameliorates the effect of the higher rate of incomplete denervation associated with truncal vagotomy. This additional protection is not available to patients who undergo truncal vagotomy and pyloroplasty. Because selective vagotomy is associated with a more complete denervation of the stomach, the antrectomy in these patients becomes unnecessary.
Pyloroplasty is both technically easier to perform and is associated with a lower mortality and that of antrectomy. Thus, it would seem that selective vagotomy and pyloroplasty could be an operation of choice in those patients who require surgical treatment for their peptic ulcer disease. The trend in recent years, however, has been to employ some form of highly selective vagotomy. The emergence of Taylor’s procedure (i.e., posterior truncal vagotomy and anterior seromyotomy that can be performed laparoscopically) has revitalized surgical options for chronic peptic ulcer disease predominately in Europe and Asia. In the United States, where most of the ulcer operations are performed for emergent indications of bleeding or perforation, the most common procedures are omental patching (laparoscopic or open procedure) or truncal vagotomy with pyloroplasty. Despite this, selective vagotomy should remain in the minds of the gastrointestinal surgeons when faced with surgical indications for treating peptic ulcer disease.
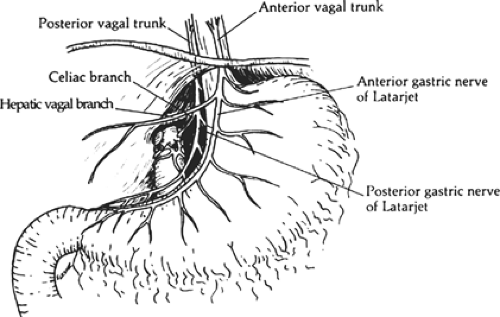
The two major vagal trunks descend along the esophagus and emerge through the esophageal hiatus of the diaphragm as anterior and posterior branches (Fig. 1). The anterior branch originates from the left side and gives off the hepatobiliary and pancreatic branches. This anterior branch continues along the lesser curvature of the stomach in the anterior leaflet of the gastrohepatic omentum as the anterior nerve of Latarjet. The posterior branch originates from the right side and ultimately gives rise to the celiac branch. This celiac branch provides parasympathetic innervation to the pancreas, duodenum, small intestine, and right colon; this posterior branch continues in the posterior leaflet of the gastrohepatic omentum as the posterior nerve of Latarjet. There is a plexus of fibers that connect the right and left vagus nerves. Furthermore, many patients manifest their major trunks of the vagus nerves as multiple branches. This fact alone is likely responsible for the incomplete denervation of the stomach during truncal vagotomy. Simply finding the two major branches is insufficient when performing a vagotomy. The goal of selective vagotomy is to completely divide all the vagal branches entering the stomach and preserve those branches to the liver and pancreas biliary tree and intestines.
The patient is prepared for general endotracheal anesthesia. Preoperative prophylactic antibiotics and deep venous thrombosis prophylaxis are administered. Nasogastric catheter and urinary catheters are placed. An upper midline incision is made from the xiphoid to the umbilicus. Occasionally, patients may require the excision to be extended past the umbilicus. Alternatively, a chevron-type incision may be used but there is no specific advantage to this. In order to achieve the optimal exposure, it is useful to bring the upper portion of the incision up along the left side of the xiphoid. A Bookwalter retractor is
fixed to the table frame. A round ring is placed, with emphasis to elevate the rib cage for best exposure. The abdomen is examined in a systematic fashion. The duodenum and stomach should be carefully examined, but care should be taken to avoid overly disturbing the gastrohepatic ligament, which would make the observation of the nerves of Latarjet, hepatic, and celiac branches more difficult. Adhesions from the duodenum to the gallbladder or liver should be lysed, allowing careful inspection of the first portion of the duodenum. It is convenient to secure a nasogastric tube along the greater curvature of the stomach with Babcock clamps. This allows traction on the stomach and improves visualization of the hepatic vagal branches.
fixed to the table frame. A round ring is placed, with emphasis to elevate the rib cage for best exposure. The abdomen is examined in a systematic fashion. The duodenum and stomach should be carefully examined, but care should be taken to avoid overly disturbing the gastrohepatic ligament, which would make the observation of the nerves of Latarjet, hepatic, and celiac branches more difficult. Adhesions from the duodenum to the gallbladder or liver should be lysed, allowing careful inspection of the first portion of the duodenum. It is convenient to secure a nasogastric tube along the greater curvature of the stomach with Babcock clamps. This allows traction on the stomach and improves visualization of the hepatic vagal branches.
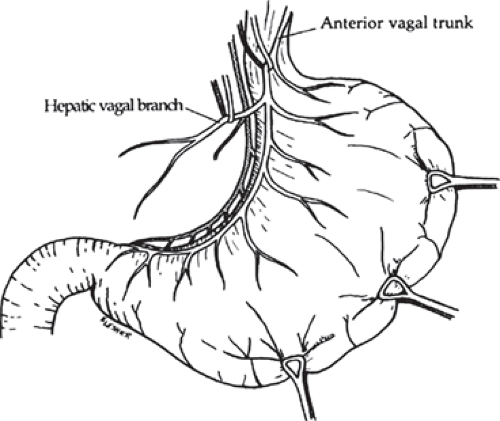
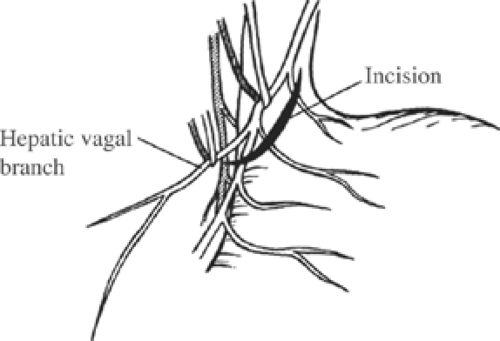
The peritoneum overlying the esophagus is opened sharply. Fibrofatty tissue is swept away using a “peanut” or similar device with vertical motions along the anterior esophagus. With caudad traction on the stomach, the anterior esophagus is palpated until the anterior vagal trunk is identified. The vagal nerve under traction has been likened to a banjo string. Once this is identified, a vessel loop should be placed around the nerve. The hepatic branch is then identified by placing gentle traction on the anterior vagal trunk and the stomach. Once identified, the hepatic vagal branch is encircled with a loop (Fig. 2). It is important to identify the junction of the hepatic branch in the anterior vagus nerve. Once accomplished, all tissues to the left between these branches and the lesser curve of the stomach are divided, with specific care to preserve the hepatic vagal branches (Fig. 3). Retraction of the esophagus to the left with a narrow ribbon retractor will generally reveal the posterior vagus nerve. It is often directly anterior to the aorta. If one chooses to encircle the esophagus with a Penrose drain, care must be taken to avoid injury to the posterior nerve as well. The celiac branch can then be palpated by a finger, and feels like a taught string that extends toward the celiac plexus.
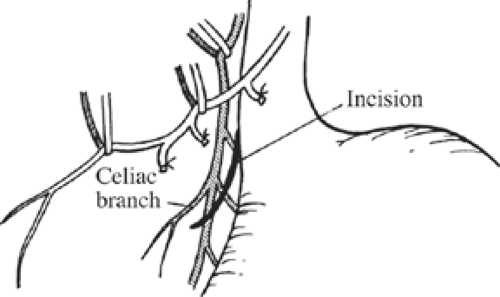
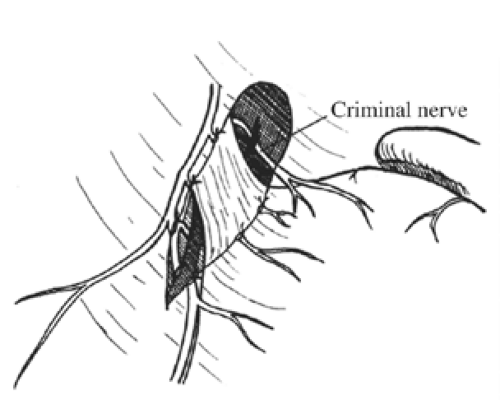
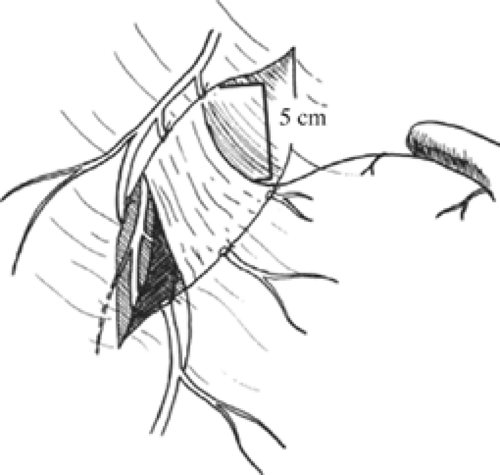
Once these branches are identified, all tissues between these nerve trunks and lesser curvature of the stomach are divided (Fig. 4). Students of anatomy will recognize the necessity to ligate the ascending branch of the left gastric artery during this maneuver. The lesser curvature of the stomach should be completely free. Because achieving a low recurrence rate of ulcer disease is partly dependent on the completeness of vagotomy, the surgeon must search for any remaining vagal branches that have arisen above the esophageal hiatus. The well-named criminal nerve of Grassi, if present, can be found generally along the left lateral wall of the esophagus, entering the stomach cephalad to the first short gastric vessel (Fig. 5). Placing gentle caudad traction on the esophagus and palpating along the left side of the esophagus for an additional “bowstring” will reward the surgeon with the nerve’s discovery. Failure to discover this nerve may result and also recurrence or a failure to achieve healing. In order to be certain that an adequate vagotomy has been performed, all tissues surrounding the esophagus should be cleared away for several centimeters above the gastroesophageal junction (Fig. 6). Classically, a very superficial incision is made circumferentially around the esophagus to divide fine nerve fibrils that run proximally to the cardia (Fig. 7). Alternatively, the esophagus is elevated by placing the surgeon’s hand
beneath it and using a nerve hook in the other hand to identify, elevate, and divide these small nerve fibers.
beneath it and using a nerve hook in the other hand to identify, elevate, and divide these small nerve fibers.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree