Secretory Carcinoma
FREDERICK C. KOERNER
Many of the histologic patterns of breast carcinoma that occur in adults have also been reported in patients younger than 20 years.1 Secretory, or juvenile, carcinoma is often found in children, but the majority of cases have been reported in adults2 (Fig. 22.1). Consequently, the term “secretory” is preferable to “juvenile” when referring to these neoplasms. The cellular characteristics of the lesion are identical in patients of all ages.
CLINICAL PRESENTATION
Age
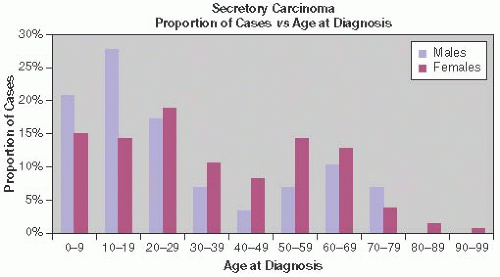
Secretory carcinoma was first fully described in 1966 by McDivitt and Stewart3 in a report on seven patients, whose ages ranged from 3 to 15 years and averaged 9 years. Six years later, Oberman and Stephens4 reported the occurrence of secretory carcinomas in two women 25 and 56 years old, and Oberman5 subsequently described four more examples in adult women 22 to 73 years old. Descriptions of many additional cases in both children and adults have been published since, mostly as single case reports or series composed of just a few patients. It has become clear that secretory carcinoma affects individuals throughout life. The reported ages of women with secretory carcinoma range from 3 to 91 years.3,6,7 Several reports document carcinomas in girls aged 5 years or younger.8,9,10,11,12 Male patients exhibited a similar age range (3 to 79 years)13,14,15 and included a tumor in a 6-year-old boy.16 The pediatric and adolescent age groups seem to account for a greater proportion of the secretory carcinomas seen in males. About one-half of the patients were beyond the age of 20 years, with a substantial number between 50 and 69 years (Fig. 22.1). Young patients are approximately evenly divided among children and those in their teens, but there seems to be a dearth of reports of individuals between the ages of 10 and 15 years.
Clinical Findings
Secretory carcinoma may occur in any part of the breast. Subareolar lesions have been associated with nipple discharge, but in most cases the patient describes a painless, circumscribed mass that may have been present for 1 or more years.5,8,9,10 A subareolar tumor is most common in prepubertal girls and males because their breast tissue is localized in this region, but even among women, the central region of the breast stands out as a favored location. Secretory carcinoma usually grows as a single mass. Rare carcinomas present as two or more nodules evident on radiologic studies or macroscopic examination.8,17,18,19
Imaging Studies
Mammography typically reveals a discrete tumor with smooth or irregular borders.2,10,17,19,20,21,22,23 A secretory carcinoma with a rounded contour can be mistaken for a fibroadenoma (FA) or a papilloma on imaging studies, especially in a young patient. Mammograms exhibit calcifications in rare cases only.2 Sonography discloses a solid, hypoechoic to isoechoic mass, which may have a microlobulated border.10,17,20,24,25,26,27 Mun et al.28 described and illustrated the sonographic features of six secretory carcinomas in adult women. Radiologic studies are not commonly used in the evaluation of breast tumors in children; so the literature does not offer secure information regarding the imaging features of secretory carcinoma in this age range.
Unusual Clinical Presentations
Several cases of secretory carcinoma involve unusual clinical circumstances. For example, Paeng et al.24 reported a 31-year-old woman with a history of acute myelogenous leukemia who developed secretory carcinoma while in complete remission after treatment with bone marrow transplantation and chemotherapy. Shin et al.29 described a 46-year-old woman who developed secretory carcinoma in axillary breast tissue. The mass, which occasionally secreted fluid, had been present for 8 years before the carcinoma was diagnosed. Brandt et al.30 recounted the case of a 13-year-old girl with a secretory carcinoma in the axilla. This example seemed to have arisen from cutaneous adnexal glands rather than from axillary breast tissue. Secretory carcinoma developed in the breast of a male-to-female transgender individual, who had undergone “long-term cross-sex hormone treatment” of an unspecified nature.31 The tumor expressed the ETV6–NTRK3 fusion gene that has been detected in most mammary secretory carcinomas.
No clinical evidence of a hormonal abnormality has been described that would explain the secretory properties of the tumor. Pregnancy has not been implicated in the development of secretory carcinoma. One pregnant patient noted a tumor that proved to be secretory carcinoma when excised 4 years later.32 Associated breast conditions have been described in a few cases. Gynecomastia accompanied a minority of the secretory carcinomas in male patients. A 23-year-old man with secretory carcinoma reported that a small breast nodule present since the age of 2 years had been ascribed to gynecomastia.33
The coexistence of juvenile papillomatosis and secretory carcinoma has been described,9,34,35,36 but the evidence presented to substantiate the diagnosis of juvenile papillomatosis is not convincing in all instances. For example, Tokunaga et al.36 described a 13-year-old girl who succumbed to secretory carcinoma arising in the left breast. At autopsy, a proliferative lesion was found in the right breast. The diagnosis given to the proliferation in the right breast was “juvenile cystic papillomatosis,” but the illustration provided shows a ductal epithelial proliferation more appropriately termed “juvenile papillary duct hyperplasia.” A second case presented by these authors as secretory carcinoma in “juvenile papillomatosis” appears from the illustrations to be cystic transformation of secretory carcinoma.
GROSS PATHOLOGY
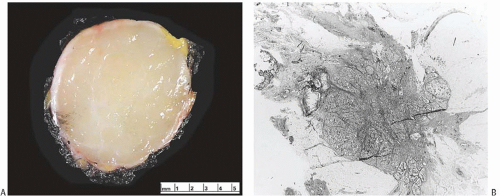
On gross examination, secretory carcinoma usually forms a circumscribed, firm mass, which may be lobulated (Fig. 22.2). The tumors tend to be 3 cm or less in diameter, although a carcinoma spanning 12.5 cm was reported in a man.37 Among six women in one report, the mean tumor size was 1.7 cm (range 0.5 to 4.0 cm)38; in another publication,39 it was 5.5 cm (range 2.5 to 10 cm) in seven female patients and one male patient 17 to 60 years old. Rarely the tumor has infiltrative margins. Various colors have been mentioned, usually shades of white to gray or tan to yellow. Observers noted that one secretory carcinoma appeared “… grayish-white to tan in color and had a center that varied between a spongy consistency and a microcystic nature. The margin of the lesion was ill defined. During sectioning, a colorless viscous secretion emerged from the central tissue compartment.”31
MICROSCOPIC PATHOLOGY
Like other forms of ductal carcinoma, secretory carcinoma often has an intraductal component. All six secretory carcinomas reported by Lae et al.38 had accompanying ductal carcinoma in situ (DCIS), and Kameyama et al.22 described
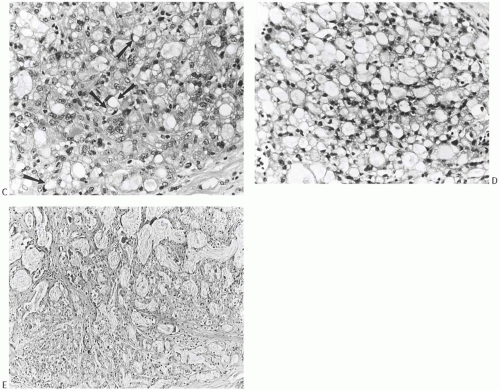
a purely noninvasive form of secretory carcinoma. The in situ component of secretory carcinoma can exhibit certain of the growth patterns seen in the conventional types of ductal carcinoma. Most commonly, the DCIS is papillary (Fig. 22.3A) or cribriform,22 but solid foci (Fig. 22.3B, C) and, rarely, comedonecrosis may also be found. These features are seen in the invasive components, which tend to be relatively compact with papillary, microcystic, and glandular patterns (Fig. 22.4). Lobulation evident on macroscopic examination usually results from fibrous septa within the mass (Fig. 22.5). The borders of the carcinoma are usually circumscribed microscopically, but overtly infiltrative growth is sometimes present (Fig. 22.6). Microcalcifications are rarely seen in the neoplastic glands or in the stroma. The tumor cells vary from secretory to apocrine in their features. Cells of a secretory nature possess pale to clear, pink or amphophilic cytoplasm that contains abundant secretion. The nuclei appear low-grade, and they vary from small to modest in size. The chromatin ranges from dark and finely dispersed to pale and granular. Nuclei with pale chromatin usually contain small, uniform nucleoli (Fig. 22.7). Cells with apocrine features contain granular eosinophilic cytoplasm and nuclei characteristic of apocrine cells40,41 (Fig. 22.8). One usually finds both types of cell in a carcinoma, although one type or the other can predominate. On occasion, cells with apocrine features growing in a solid pattern comprise most of the tumor and thereby obscure the secretory nature of the carcinoma (Fig. 22.9). The cells do not display noticeable mitotic activity or necrosis.
a purely noninvasive form of secretory carcinoma. The in situ component of secretory carcinoma can exhibit certain of the growth patterns seen in the conventional types of ductal carcinoma. Most commonly, the DCIS is papillary (Fig. 22.3A) or cribriform,22 but solid foci (Fig. 22.3B, C) and, rarely, comedonecrosis may also be found. These features are seen in the invasive components, which tend to be relatively compact with papillary, microcystic, and glandular patterns (Fig. 22.4). Lobulation evident on macroscopic examination usually results from fibrous septa within the mass (Fig. 22.5). The borders of the carcinoma are usually circumscribed microscopically, but overtly infiltrative growth is sometimes present (Fig. 22.6). Microcalcifications are rarely seen in the neoplastic glands or in the stroma. The tumor cells vary from secretory to apocrine in their features. Cells of a secretory nature possess pale to clear, pink or amphophilic cytoplasm that contains abundant secretion. The nuclei appear low-grade, and they vary from small to modest in size. The chromatin ranges from dark and finely dispersed to pale and granular. Nuclei with pale chromatin usually contain small, uniform nucleoli (Fig. 22.7). Cells with apocrine features contain granular eosinophilic cytoplasm and nuclei characteristic of apocrine cells40,41 (Fig. 22.8). One usually finds both types of cell in a carcinoma, although one type or the other can predominate. On occasion, cells with apocrine features growing in a solid pattern comprise most of the tumor and thereby obscure the secretory nature of the carcinoma (Fig. 22.9). The cells do not display noticeable mitotic activity or necrosis.
Secretion accumulates in the tumor cells, in the glands formed by the tumor cells, and in the microcystic spaces associated with the tumor cells. The secretory material appears pale pink or amphophilic with hematoxylin and eosin (H&E) staining, and it often contains lacunae, which create a “bubbly” appearance. The secretion stains with the periodic acid-Schiff (PAS) and Alcian blue methods. PAS staining persists after diastase digestion (Fig. 22.10A), and Alcian blue staining persists after sialidase digestion. The secretory material stains with toluidine blue at pH 1.5 and reacts variably for mucin. These findings indicate that the secretory material contains sulfated mucopolysaccharides and sialomucin.42 The material displays a purple or violet color with the crystal violet stain. The secretion in microcystic areas (Fig. 22.4B) resembles the material that accumulates in cystic hypersecretory lesions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree