Salivary Glands
Abdelmonem Elhosseiny
ANATOMIC AND HISTOLOGIC CONSIDERATIONS
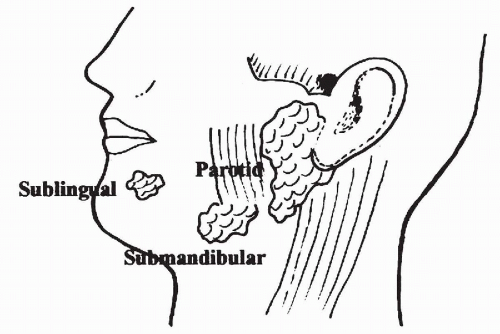
There are two groups of salivary glands, major and minor. The major salivary glands comprise three large paired exocrine glands: parotid, submandibular, and sublingual (Fig. 32-1).
The parotid is the largest of the salivary glands. It is located at the angle of the mandible and encircles the ear lobe. It weighs approximately 25 g. The facial nerve divides the parotid into superficial and deep parts. Most neoplasms occur in the superficial part of the gland. The secretions of the parotid flow through Stensen’s duct, opening into the oral cavity on the lateral aspect of buccal mucosa. Lymph nodes are commonly present within the parotid and in the periparotid region.
The submandibular gland is approximately half the size of the parotid. It is located in the submandibular triangle between the inferior border of the mandible and the digastric muscle. Warthin’s duct carries the secretions of the submandibular gland to the floor of the mouth. There are no lymph nodes within the submandibular gland, though a few lymph nodes are adjacent to it in the submandibular triangle.
The sublingual gland is the smallest of the major salivary glands and weighs approximately 3 g. It is located in the floor of the mouth in the lingual sulcus. Secretions of the sublingual gland are carried in multiple small ducts to the oral cavity.
Several hundreds of tiny minor salivary glands are scattered throughout the mucosa of the oral and nasal cavities, larynx, and bronchial tree. They are most abundant in the posterior hard palate. The secretions of minor salivary glands are carried to the oral cavity by short excretory ducts (Ellis and Auclair, 1996).
Ectopic Salivary Gland
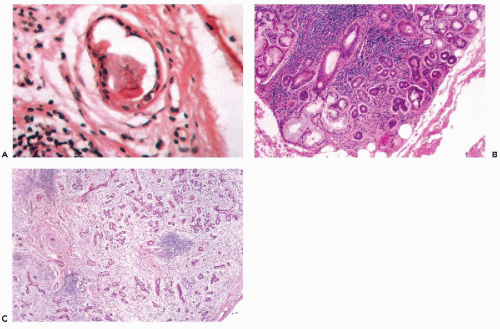
Ectopic salivary gland tissue is most commonly encountered as an incidental finding in periparotid and intraparotid lymph nodes (Fig. 32-2A) (Silvers and Som, 1998). These lymph nodes may play a role in the pathogenesis of Warthin’s tumor and are a potential site of inflammatory lesions or metastases from tumors in adjacent and distant locations (Ellis and Auclair, 1996). Less commonly, ectopic salivary
tissue can be seen in soft tissues along the anterior border of the sternocleidomastoid muscle and in cervical lymph nodes (Lassaletta-Atienza et al, 1998). Rarely, tumors of salivary gland origin, both benign and malignant, may develop in these sites.
tissue can be seen in soft tissues along the anterior border of the sternocleidomastoid muscle and in cervical lymph nodes (Lassaletta-Atienza et al, 1998). Rarely, tumors of salivary gland origin, both benign and malignant, may develop in these sites.
Histology
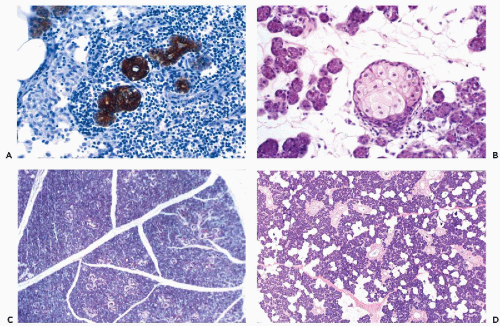
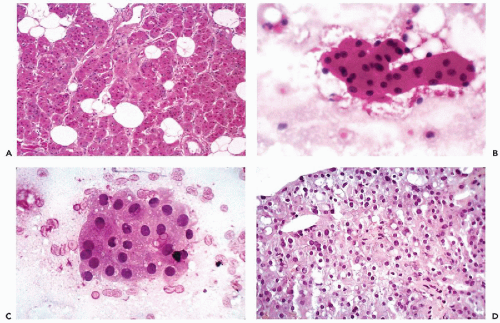
The fundamental structure of both major and minor salivary glands comprises acinar and duct units. The acini of serous, mucinous, or mixed seromucinous types are arranged in groups or lobules surrounded by a basement membrane. The cells in serous acini are approximately triangular with their narrowest part facing the luminal surface; their cytoplasm is basophilic and granular. The nuclei are round, uniform, and basally located. The mucinous cells have similar shape but their cytoplasm is clear or finely granular; the nuclei are also round and basally located. Sebaceous differentiation is sometimes noted (Fig. 32-2B) and well-formed sebaceous glands have been reported in 10% to 42% of normal salivary glands (Martinez-Madrigal et al, 1997). Saliva that is formed in the acini flows through a series of ducts, described below, and into the oropharyngeal cavity. The parotid is almost exclusively serous; the sublingual gland is mostly mucinous; and the submandibular gland is seromucinous. The composition of the saliva is determined by the histology of the acini. In young persons, the acinar cells are closely apposed (Fig. 32-2C) but with advancing age, the amount of interstitial adipose tissue increases while parenchymal tissue decreases (Fig. 32-2D) (Ellis and Auclair, 1996).
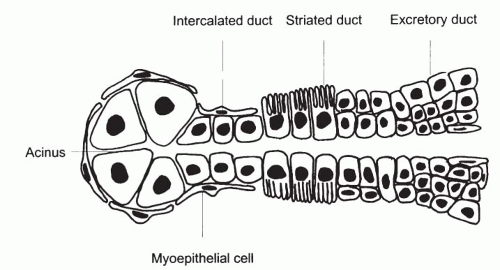
The duct system of the major salivary glands is divided into three distinct parts: the intercalated, the striated, and the excretory (interlobular) ducts (Fig. 32-3). The intercalated duct, which lies within and receives the secretions of an acinus, is very short and lined by simple cuboidal epithelium. The striated ducts are lined by columnar epithelium with basal striations and are much larger than the intercalated duct. The excretory or interlobular ducts are the terminal part of the duct system and are lined by stratified columnar epithelium that changes to squamous epithelium as the ducts approach the oral cavity. Myoepithelial cells are present at the periphery of the acini and along the outside of the intercalated ducts.
INDICATIONS FOR FINE-NEEDLE ASPIRATION
Fine-needle aspiration (FNA) cytology has now been accepted by head-and-neck surgeons as an excellent, though challenging, primary method of evaluating space-occupying lesions of the salivary glands (Zaijcek, 1974; Batsakis et al, 1992; Boccato et al, 1998). Used correctly, it can provide a definitive diagnosis when clinical findings and radiographic studies are not adequate to distinguish nonneoplastic from neoplastic lesions, or benign from malignant tumors. It has advantages over an operative incisional biopsy, which has the potential risk of fistula formation and, in the case of malignant neoplasms, the theoretical possibility of seeding tumor cells.
FNA of salivary glands generally address the following questions:
Is the mass of salivary gland origin?
If the mass is of salivary gland origin, is it neoplastic or non-neoplastic?
If the mass is neoplastic, is it benign or malignant?
If the mass is malignant, is it primary or metastatic?
If primary, what is the tumor type?
If metastatic, what is the site of origin?
The diagnosis rendered by FNA often influences management of the patient (Zaijcek, 1974; Kocjan et al, 1990; Orell, 1995) and allows for appropriate treatment planning. For example, if a tumor is benign, surgical intervention may be delayed or modified, whereas a malignant neoplasm may call for prompt surgical treatment or irradiation. The diagnosis of lymphoma calls for still other investigative and treatment options.
Lesions of the skin and subcutaneous tissues overlying the salivary gland, for example, epidermal inclusion cysts, lipomas, tumors of sweat glands, basal cell carcinomas, or schwannoma, may be mistaken for a salivary gland tumor (see below). It should also be noted that an enlargement of a salivary gland may be caused by a nonneoplastic cyst, reactive intraparotid lymph node, stones, or sialadenitis.
As mentioned earlier, lymph nodes within the parotid or peri-parotid region may be the site of metastatic tumor, lymphoma, and inflammatory or reactive processes.
Complications of FNA of Salivary Gland
Significant complications of fine-needle aspirates of salivary gland are rare. Kern (1988) described postaspiration necrosis in a case of Warthin’s tumor and Layfield et al (1992) described similar occurrences in cases of pleomorphic adenoma. Stephen et al (1999) reported xanthogranulomatous sialadenitis following needle aspiration of Warthin’s tumor. Li et al (2000) and Mukunyadzi et al (2000) reviewed the histology of a large number of salivary gland lesions previously sampled by FNA. They observed that areas of infarction, necrosis, hemorrhage, inflammation, and granulation tissue were quite common but did not interfere with the final histologic diagnosis. A potential source of error is the presence of exuberant squamous metaplasia of ducts, apparently induced by aspiration, which in some cases was marked enough to mimic a mucoepidermoid or squamous cell carcinoma. Obviously, knowledge of a prior aspiration procedure may be valuable in the interpretation of tissue samples. There has been no report known to us of tumor implantation following salivary gland aspiration.
CYTOLOGY OF NORMAL SALIVARY GLANDS
It is important to be familiar with cytologic features of normal salivary glands, which may be observed in needle aspirates of a prominent but normal salivary gland or failure to sample the target lesion.
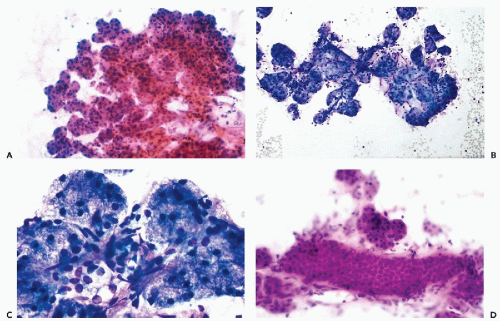
The typical aspirate is composed of acinar cells, duct cells, and adipose tissue. Acinar cells form cohesive, spherical groups composed of polyhedral cells with vacuolated or granular cytoplasm and small, uniform nuclei (Fig. 32-4A-C). An entire lobule is often present with intervening adipose tissue. The duct cells form either flat honeycomb sheets of small, uniform cuboidal cells with centrally located round nuclei, or tight tubular structures composed of similar cells (Fig. 32-4D).
NONNEOPLASTIC LESIONS
Cystic lesions
Cysts may involve acinar tissue of the salivary glands or their ducts; they are more frequently encountered in minor salivary glands.
Mucocele
A mucocele is a mucus-filled cyst, in or around a minor salivary gland, caused by rupture of the gland with extravasation of mucus into adjacent tissues. The cyst has a fibrous wall lined by granulation tissue, and contains macrophages as well as mucus. Mucoceles present clinically as a raised, painless, soft or fluctuant nodule. They may arise anywhere in the oral cavity, but the lower lip is the most common site.
Retention Cyst
Retention cysts are closely related to mucoceles, but much less common. They are encountered more frequently in major salivary glands, particularly the parotid, and result from duct obstruction by microliths or inspissated secretions (Fig. 32-5). Patients are usually older than those affected by mucocele. Retention cysts are true cysts with an epithelial lining that may be columnar, squamous, or mucus-secreting. The most important consideration in the differential diagnosis is a low-grade mucoepidermoid carcinoma. Jayaram et al (1998) reported an erroneous diagnosis of squamous carcinoma in a patient with atypical squamous metaplasia of a cyst formed by dilated salivary duct.
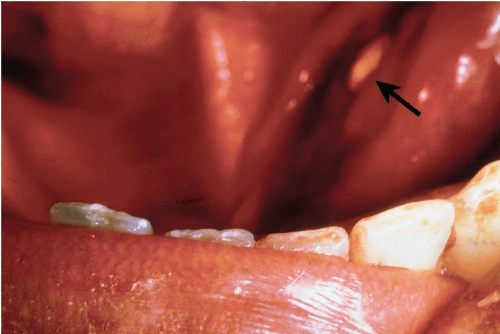 Figure 32-5 Impacted stone. Floor of mouth of a patient with stone impacted at the orifice of Warthin’s duct of the submandibular gland, just to the left of the frenulum of the tongue (arrow). |
Cytology
An aspirate of a mucocele or retention cyst yields clear or mucoid fluid. The cell content is scanty, but there are usually a few macrophages, with or without other inflammatory cells, and a few epithelial cells. Rarely, metaplastic oncocytic cells may be present but they are few in number and not associated with lymphocytes, as in Whartin’s tumor (see below). Normal salivary gland tissue may be seen if the aspirating needle has passed through normal gland. If the cyst collapses completely following aspiration, the aspiration may be considered as a curative procedure. It is very important to verify that there is no residual mass after aspiration; its presence indicates that a solid tumor is hiding behind the cyst and repeat aspiration or biopsy are strongly recommended. If a cyst recurs multiple times, surgical excision is the treatment of choice (Zarka, 1996).
Benign Lymphoepithelial Cysts
These cysts are typically seen in adults and most are unilateral. They present within, or around, the parotid and are usually well circumscribed. Previously rare, such cysts have been observed with increasing frequency in patients with acquired immunodeficiency syndrome (AIDS). The HIV-associated lymphoepithelial cysts are more frequently bilateral and often accompanied by diffuse cervical lymphadenopathy. Stigmata of AIDS may be evident, including Kaposi’s sarcoma, oropharyngeal herpes, or monilial infections. The cysts are lined by squamous epithelium and surrounded by dense lymphoid tissue with reactive lymphoid follicles (Wiedner et al, 1986). The histologic structure and cytology of the HIV-associated cysts are identical to the benign lymphoepithelial cysts (Fig. 32-6) (Finfer et al, 1990).
Cytology
Aspirates contain an abundant, heterogeneous population of reactive lymphocytes and macrophages in clear cyst fluid. In addition, there may be a few small clusters of squamous or sometimes columnar or cuboidal epithelial cells (Elliot and Oertel, 1990). Lopez-Rios et al (1999) reported the presence of crystalloids identified as crystallized amylase in cyst fluid from two such patients. For further discussion of crystalloids in salivary gland lesions, see below. Vicandi et al (1999) reported the presence of numerous multinucleated giant cells in two HIV-infected patients. The presence of viral protein could be demonstrated in these cells by immunocytochemistry with p24.
Nonneoplastic Solid Lesions
Acute Sialoadenitis
Acute inflammatory lesions of salivary glands are commonly caused by stasis of secretions with microliths (Fig. 32-7A) and bacterial infection, or may be caused by cytomegalovirus (CMV) or mumps (Wax and Layfield, 1994). Patients with acute sialoadenitis present with tenderness and diffuse enlargement of the affected gland.
Cytology
Aspiration is rarely necessary to make the diagnosis but if performed, it yields numerous polymorphonuclear leukocytes, frequently associated with cellular debris, macrophages, and proteinaceous background. Fragments of regenerative acinar or ductal tissue may be present.
Chronic Sialoadenitis
Chronic inflammation of salivary gland is either the result of duct obstruction caused by stones (sialolithiasis), or by physical, microbial, or immunological injuries. It is a common complication of radiotherapy of head and neck cancers and may result in xerostomia (dry mouth).
The end stage of chronic sialoadenitis is atrophy of the acini and stromal fibrosis, usually resulting in a firm gland that clinically may simulate a tumor (Fig. 32-7B,C) (Chai et al, 1997).
Cytology
Aspirates of salivary gland, with chronic sialoadenitis, are generally scanty and contain a few lymphocytes, plasma cells, some neutrophils, cellular debris, and macrophages in a background of mucus. Rarely, fragments of glands or of fibrous tissue will be present in the aspirate. In cases of sialolithiasis, cystic dilatation of ducts may occur. Such ducts may show marked proliferation of ductal epithelium resulting in formation of mucoid material. Squamous metaplasia of the epithelium may also occur. An aspiration smear in such cases may show calcified debris of stones and atypical squamous cells in the background of mucus, sometimes mimicking mucoepidermoid carcinoma (Stanley et al, 1996). A case of chronic sialadenitis with numerous psammoma bodies mimicking cancer was described by Frierson and Fechner (1991).
Granulomatous Sialoadenitis
This form of chronic sialoadenitis may have several possible causes, including tuberculosis and sarcoidosis (Aggarwal et al, 1989). Duct obstruction, caused by a stone or tumor, is one potential cause of granulomatous inflammation. Xerostomia is uncommon in unilateral disease and in the absence
of lithiasis, these patients may be asymptomatic. In sarcoidosis, the granulomas are usually bilateral, frequently involve surrounding lymph nodes, and may involve other organs, including lung (see Chap. 19). Unfortunately, many cases of granulomatous sialoadenitis remain without identifiable cause.
of lithiasis, these patients may be asymptomatic. In sarcoidosis, the granulomas are usually bilateral, frequently involve surrounding lymph nodes, and may involve other organs, including lung (see Chap. 19). Unfortunately, many cases of granulomatous sialoadenitis remain without identifiable cause.
Cytology
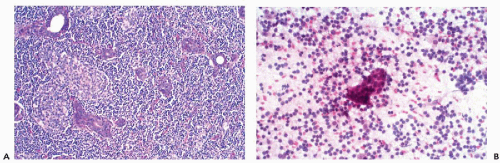
The granulomas are focal and may be difficult to sample. If representative material is obtained, however, the smears may sometimes contain whole granulomas (Fig. 32-8A). More often, however, the smears show aggregates of carrot-shaped, spindle epithelioid cells and sometimes well-formed giant cells in a background of lymphocytes and plasma cells (Fig. 32-8B,C). Special stains and bacterial culture may be needed to search for microorganisms if tuberculosis or other infectious agents are suspected.
Benign Lymphoepithelial Lesions
Myoepithelial Sialoadenitis
These benign lymphoepithelial autoimmune lesions are characterized by a progressive, destructive lymphocytic infiltrate of salivary (and lacrimal) glands resulting in loss of acini (Fig. 32-9A). There is proliferation of myoepithelial cells with preservation of the ducts, leading to the formation of epithelial-myoepithelial islands; hence, the name of myoepithelial sialoadenitis that is now attached to this disorder.
The lesion is closely associated with Sjögren’s syndrome, in which the affected individuals experience dryness of the mouth and eyes (xerostomia and xerophthalmia), caused by the autoimmune destruction of salivary and lacrimal glands. It may occur in association with other auto-immune disorders such as rheumatoid arthritis, systemic lupus erythematosus, scleroderma, or polymyositis.
Cytology.
Aspiration smears show small tight clusters of epithelial-myoepithelial cells within a heterogeneous population of lymphocytes and occasional tingible body macrophages (Fig. 32-9B). Rare microcalcifications may be present. The epithelial-myoepithelial cells may be difficult to find or to identify in smears. In such cases, the diagnosis may be suspected based on the history and nature of the inflammatory reaction, but difficult or impossible to prove by cytology.
Necrotizing Sialometaplasia
A necrotizing, often ulcerative, sialoadenitis most commonly affects minor salivary glands of the palate. It is a self-limited disease of unknown etiology. Histologically, lobular necrosis and sialoadenitis are accompanied by squamous metaplasia of the ducts or acini (Brannon et al, 1991).
Cytology.
An aspirate yields islands of metaplastic squamous epithelium within a background of mucus with few inflammatory cells. Mucin-secreting epithelial cells
may be present and squamous cells may show some inflammatory atypia. Careful history and the typical clinical presentation are important in avoiding an erroneous diagnosis of squamous or mucoepidermoid carcinoma.
may be present and squamous cells may show some inflammatory atypia. Careful history and the typical clinical presentation are important in avoiding an erroneous diagnosis of squamous or mucoepidermoid carcinoma.
BENIGN NEOPLASMS
Most tumors of the salivary glands are benign. The principal types of these tumors are listed in Table 32-1.
Pleomorphic Adenoma (Benign Mixed Tumor)
Clinical Data
Pleomorphic adenomas (PA) are the most common salivary gland tumors; they constitute 60% to 70% of all parotid tumors, approximately 50% of tumors in the submandibular glands, and 40% to 70% of all tumors of the minor salivary glands.
Ninety percent of the parotid tumors occur in the superficial lobe. The two most common sites for PA arising in minor salivary glands are the lip and palate. Rarely, PA may
arise from ectopic salivary tissue at unusual sites, as for example in cervical lymph nodes and soft tissue of the neck, the breast, the lacrimal glands, or skin of upper and lower extremities. The skin lesions are of sweat gland origin and are usually referred to as chondroid syringoma (Surana et al, 1993; Lassaletta-Atienza et al, 1998).
arise from ectopic salivary tissue at unusual sites, as for example in cervical lymph nodes and soft tissue of the neck, the breast, the lacrimal glands, or skin of upper and lower extremities. The skin lesions are of sweat gland origin and are usually referred to as chondroid syringoma (Surana et al, 1993; Lassaletta-Atienza et al, 1998).
TABLE 32-1 PRINCIPAL BENIGN TUMORS OF SALIVARY GLANDS | |||||
|---|---|---|---|---|---|
|
PA typically presents as a discrete, painless, firm, mobile mass in a major salivary gland (Fig. 32-10A), or as a painless submucosal nodule in a minor salivary gland. A tumor of the parotid or the submandibular gland may be mistaken clinically for an enlarged lymph node and vice-versa.
A preoperative diagnosis of PA is of significant clinical value as it should alert the surgeon that the tumor must be carefully removed with its capsule intact. Incomplete removal may result in a recurrence of the tumor in the form of multiple, disfiguring subcutaneous nodules, sometimes many years after surgery.
On the rarest occasions, PA may form metastases to neck lymph nodes, lung, and other organs. Such tumors cannot be distinguished morphologically from the nonmetastasizing variety (Gerughty et al, 1969; Spiro et al, 1977; Chen, 1987).
Histology
PA is a tumor of diverse histologic appearance. It comprises a mixture of epithelial, myoepithelial, and stromal mesenchymal components (Fig. 32-10B). The presence and the prominence of each component may vary in different parts of the same tumor, and from tumor to tumor. Some pleomorphic adenomas are very cellular, composed of sheets of epithelial cells with only scanty stroma; in others stroma predominates. The epithelial cells may form solid sheets of cells, tubules, ducts, acini, or trabecula. Foci of squamous metaplasia, with or without keratin formation, oncocytic metaplasia, mucus gland formation, or sometimes sebaceous differentiation may occur within the epithelial structures. The myoepithelial cells produce the mesenchymal stromal component that may be myxoid, chondroid, or hyalinized. Blending of the epithelial structures with the stroma is one of the most characteristic features of this tumor.
The myoepithelial cells in the chondromyxoid stroma are typically stellate or round, but in cellular areas, they may be spindly, plasmacytoid, or polygonal in appearance (Ellis and Auclair, 1996). The nuclei of spindly myoepithelial cells are generally hyperchromatic and tapered. Plump, spindle-shaped myoepithelial cells with eosinophilic cytoplasm can resemble cells of leiomyoma. The myoepithelial cells may also form small clusters mimicking epithelial cells.
Tyrosine crystals were initially described by Bottles et al (1984) in a case of pleomorphic adenoma. In other PAs, oxalate and yellow hippurate crystals have been identified (see below).
Cytology
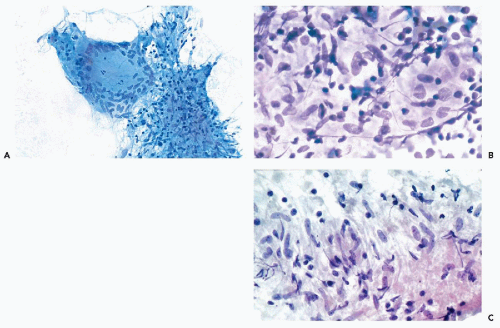
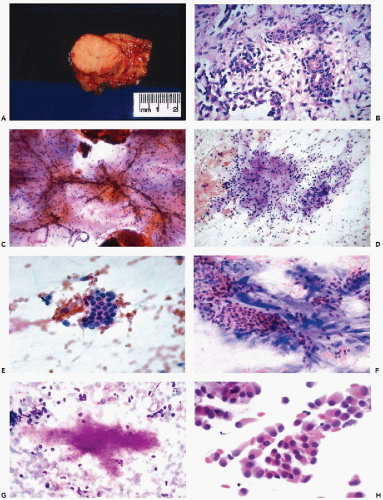
The cytologic features of PA are usually quite characteristic and the correct diagnosis can be readily established on an adequate specimen in most cases (Koss et al, 1992; Dardick et al, 1999). Although the aspirate is usually rich in cells, it is the presence of the chondromyxoid stroma, often containing capillary vessels, that is of critical diagnostic value (Fig. 32-10C). The stromal cells are slender, spindly, or stellate mesenchymal cells that may be found singly or in clusters (Fig. 32-10D). They commonly merge imperceptibly with epithelial cells, but epithelial cells may be entirely separate (Fig. 32-10E). The chondromyxoid stroma forms irregularly shaped structures that stain gray-green in Papanicolaou stain (Fig. 32-10F), or intensely red or purple with hematologic stains (Fig. 32-10G). Cellularity is variable, as is the ratio of epithelial cells to stroma, with some tumor fragments composed mainly of stroma and others formed mainly by epithelial cells (Frable and Frable, 1991).
The epithelial cells usually form loosely cohesive clusters that are sometimes of papillary configuration, but may also be arranged in flat sheets or sometimes tubules, and are typically intermixed with the chondromyxoid stroma (Fig. 32-10F). When in sheets, the epithelial cells are of equal size with scanty, pale cytoplasm and round or slightly oval nuclei with fine, evenly textured chromatin (Fig. 32-10E). Murty and Sodhani (1993) reported the presence of intranuclear cytoplasmic inclusions. Occasional larger epithelial cells, with well-defined eosinophilic cytoplasm and eccentric small nuclei, may be observed, but nuclear chromatin is finely granular and evenly distributed, often with tiny nucleoli (Klijanienko and Vielh, 1996). Also, in rare instances, epithelial cells with basaloid features are arranged in ball-like structures, as commonly observed in adenoid cystic carcinoma (see below) (Elshiekh and Bernacki, 1996).
The myoepithelial cells may form clusters of loosely cohesive cells. Individual cells may have a spindle or plasmacytoid appearance with indistinct cytoplasmic borders (Fig. 32-10H). Such cells cannot be readily differentiated from epithelial cells.
Various types of metaplasia that have been observed in PA, as described above, are seldom evident in the needle aspirate. When either squamous, oncocytic, sebaceous, or mucinous metaplasia is prominent, the possibility of a low-grade mucoepidermoid carcinoma must be considered (Stanley and Löwhagen, 1990). Rarely, aspirates of benign PA have been reported to contain calcifications resembling psammoma bodies (Qizilbash et al, 1985).
When present, crystalloids are useful in confirming the diagnosis of PA. Several different varieties of crystals have been identified. Tyrosine crystalloids usually form yellow or pink leaf-shaped structures (Fig. 32-11A,B) but may also form needle-shaped and tubular crystals (Lopez-Rios et al, 1999). Other crystalloids are the polygonal, yellow-staining hippurate crystals (Fig. 32-11C), needle-shaped oxalate crystals, radially arranged needle-shaped collagenous crystals and multifaceted amylase crystals with pointed ends (Campbell et al, 1985; Sugihara et al, 1998). Only tyrosine crystals have been reported in low-grade adenocarcinomas of salivary glands (Raubenheimer et al, 1990; Lopez-Rios, 1999). So far as is known today, all other crystalloids have been observed only in benign PA.
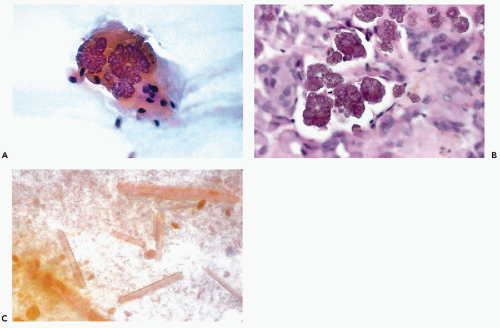 Figure 32-11 Crystalloids in benign pleomorphic adenomas. A. Leaf-shaped yellow tyrosine crystal in FNA of a parotid tumor. B. Similar crystals in tissue section. C. Polygonal hippurate crystals. |
Diagnostic problems in PA can arise when there is selective sampling with little or no chondromyxoid ground substance and the lesion may be interpreted as a carcinoma. Also, the presence of many single atypical epithelial cells may raise the possibility of carcinoma ex pleomorphic adenoma. Landolt et al (1990) reported the cytologic diagnosis of a metastatic mixed tumor (PA) to the lung. The principal features of pleomorphic adenoma and its differential diagnosis are summarized in Table 32-2.
Warthin’s Tumor (Adenolymphoma)
Warthin’s tumor, also known as papillary cystadenoma lymphomatosum, or adenolymphoma, is the second most frequent benign tumor arising in the parotid.
It is a slowly growing, soft, usually cystic, painless tumor, often described as “doughy” (Fig. 32-12A). It arises almost exclusively in the lower pole of the parotid gland, and affects mainly men in the sixth or seventh decades of life. Occasionally, the tumor may arise in peri-parotid lymph nodes. The cysts, of variable sizes, contain turbid,
yellow, sticky fluid, accounting for the spongy consistency of the tumor. The tumor is multifocal or bilateral in approximately 10% of cases (Ellis and Auclair, 1996). There appears to be a close association between Warthin’s tumor and cigarette smoking (Brandwein and Huvos, 1991). No relationship has been established with Epstein-Barr virus (EBV) (van Heerden et al, 1999).
yellow, sticky fluid, accounting for the spongy consistency of the tumor. The tumor is multifocal or bilateral in approximately 10% of cases (Ellis and Auclair, 1996). There appears to be a close association between Warthin’s tumor and cigarette smoking (Brandwein and Huvos, 1991). No relationship has been established with Epstein-Barr virus (EBV) (van Heerden et al, 1999).
TABLE 32-2 PRINCIPAL CYTOLOGICAL FEATURES OF PLEOMORPHIC ADENOMA | ||||||
|---|---|---|---|---|---|---|
|
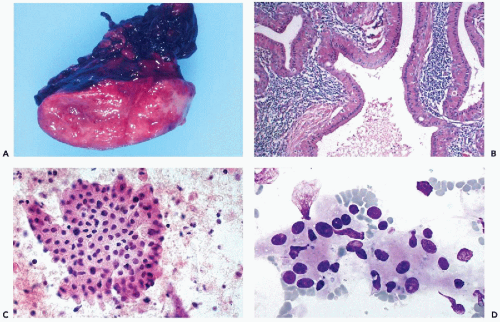 Figure 32-12 Warthin’s tumor. A. The gross appearance of a “doughy” multicystic, tan tumor, strikingly different in gross appearance and texture from the pleomorphic adenoma (mixed tumor), which is a firm, discrete, lobulated white mass (see Fig. 32-10A). The “feel” upon insertion of the needle may provide a diagnostic clue. B. Histologic sections show a multicystic tumor with oncocytic cells surfacing papillary intracystic projections of lymphoid tissue with prominent follicles, hence the alternative name “papillary cystadenoma lymphomatosum.” C,D. Thin-needle aspirate of Warthin’s tumor showing prominent oncocytic epithelial cells with abundant deeply eosinophilic cytoplasm. The oncocytic cells may form flat sheets (C), overlapping groups, or occur as single cells. The granular features of the cytoplasm are better visualized with Diff-Quik stain (D). The background of the smears is formed by precipitated cyst fluid with lymphocytes. |
Histology
Warthin’s tumor has a distinctive histologic appearance. It is composed of oncocytic epithelium, arranged in papillary fronds, lining clefts or cystic spaces. The stroma of the tumor is densely infiltrated with lymphoid tissue (Fig. 32-12B). The epithelium is arranged in two layers: inner columnar and outer cuboidal cell layers. The lymphoid stroma frequently includes reactive lymphoid follicles. In unusual cases sarcoid-like granulomas have been described (Rysska et al, 1999). Rarely, transformation into a lymphoma has been reported (Park et al, 2000). Squamous or mucinous metaplasia of the lining epithelium may
be present (Klijanienko and Vielh, 1997). The cyst contents are a mucoid, brownish material with degenerated epithelium, cellular debris, cholesterol clefts, and variable numbers of macrophages (Mandel and Tomkoria, 2000).
be present (Klijanienko and Vielh, 1997). The cyst contents are a mucoid, brownish material with degenerated epithelium, cellular debris, cholesterol clefts, and variable numbers of macrophages (Mandel and Tomkoria, 2000).
Cytology
The aspirate usually yields up to several milliliters of turbid brownish fluid; some compare it to motor oil. Smears from Warthin’s tumor typically contain three components: oncocytic cells, reactive lymphocytes, and cellular debris in a mucoid background. The most characteristic component is the many oncocytic cells, which are easily recognized as large epithelial cells with abundant, densely eosinophilic, granular cytoplasm that is rich in mitochondria (see Fig. 32-11C,D). They are arranged in flat, cohesive sheets, occasionally forming tight papillary fronds. A mixed population of reactive lymphocytes with tingible body macrophages usually forms the background of the smear. The mucoid cyst fluid usually contains cellular debris, macrophages and isolated epithelial cells with pyknotic nuclei. Not infrequently, neutrophils also are seen. Nontyrosine crystalloids have been described in the cyst contents (Nasuti et al, 2000).
The aspirates may also contain clusters of squamous cells or mucin-producing goblet cells derived from foci of squamous or mucinous metaplasia. In the presence of these components, the differential diagnosis includes branchial cleft cyst and low-grade mucoepidermoid carcinoma. Infarction of Warthin’s tumor has been reported as a rare complication of aspiration (DiPalma et al, 1999). The primary features of Warthin’s tumor and its differential diagnosis are summarized in Table 32-3.
Oncocytoma
These are rare, benign, solid tumors of salivary glands, accounting for less than 1% of all salivary gland tumors. They may occur in the parotid, submandibular, or minor salivary glands. The average age of these patients is about 70 years, and women are somewhat more often affected than men. The patients usually present with an asymptomatic swelling of a salivary gland.
TABLE 32-3 PRINCIPAL CYTOLOGICAL FEATURES OF WARTHIN’S TUMOR | ||||||
|---|---|---|---|---|---|---|
|
The tumor is usually well demarcated or encapsulated, and composed of uniform oncocytic cells arranged in solid, tubular, or trabecular patterns. The individual cells are polygonal, with characteristically abundant granular eosinophilic cytoplasm and round nuclei with eccentric nucleoli (Fig. 32-13A,D). Clear cell transformation may occur in some tumors due to glycogen deposit in the cytoplasm. However, the cell pattern remains unchanged (Brandwein and Huvos, 1991). There is no evidence of mitotic activity. Psammoma bodies have been observed in this tumor (Palmer et al, 1990).
Cytology
The aspiration smears show sheets of tightly packed polygonal oncocytic cells with abundant, deeply eosinophilic granular cytoplasm, and uniform round central nuclei with prominent slightly eccentric nucleoli. The absence of lymphocytes and cellular debris are the most important features differentiating this tumor from Warthin’s tumor (Fig. 32-13B,C). It is not known whether the very rare oncocytoid adenocarcinoma is derived from benign oncocytoma. Such a case was described by Austin et al (1987).
Oncocytosis
Oncocytosis refers to a nodular or diffuse hyperplasia of oncocytes within the salivary gland, without encapsulation or formation of a discrete mass (Palmer et al, 1990). Oncocytosis may be very extensive and replace a significant portion of the affected gland. Dardick et al (1999) pointed out that focal oncocytic metaplasia may be observed in many conditions and tumors of salivary glands. This change may be artificially induced by electrocautery (Shick and Brannon, 1998). Differentiation from oncocytoma by cytology alone is impossible (Eneroth and Zajicek, 1965). In a personally observed case, the cytologic presentation of an extensive oncocytosis was identical to an oncocytoma.
Basal Cell Adenoma
This relatively uncommon benign neoplasm has been considered a prototype of a group of salivary gland tumors collectively known as monomorphic adenomas. Because of significant confusion concerning the classification of these tumors, the term is no longer recommended by WHO (Simson, 1994) but it is still used. As defined by Seifert and Sobin (1992), basal cell adenoma is a “tumor of basaloid cells with distinct basement membrane-like material that lacks chondromyxoid stroma.”
Seventy percent of these tumors occur in the parotid, with the remainder occurring in the submandibular gland, and the minor salivary glands, especially in the upper lip.
The least common sites are the buccal mucosa and the palate. The tumor is observed more often in women, and most of the patients are elderly. Clinically, it presents as a painless, solid, mobile nodule varying in size from 2 to 3 cm (Batsakis et al, 1991).
The least common sites are the buccal mucosa and the palate. The tumor is observed more often in women, and most of the patients are elderly. Clinically, it presents as a painless, solid, mobile nodule varying in size from 2 to 3 cm (Batsakis et al, 1991).
Histology
This encapsulated tumor is composed of small basaloid cells with a peripheral palisade arrangement, mimicking basal cell carcinoma of the skin (Fig. 32-14A). Histologic patterns may be trabecular (60%), tubular (30%), solid, or membranous. In the trabecular form, the tumor cells are arranged in 3 to 4 cell-thick cords, separated by basement membrane. In the tubular type, the cells form tubules with distinct lumens resembling salivary ducts (Fig. 34-14F). The tubular or canalicular type of monomorphic adenoma more frequently involves the upper lip and is regarded by some as a special entity, but does not have any special therapeutic or prognostic significance (Zarbo et al, 2000). The membranous pattern is characterized by marked production of basement membrane-like material (Batsakis et al, 1983). Any of the monomorphic adenomas may exhibit focal oncocytic or sebaceous differentiation.
Cytology
Aspiration smears yield numerous uniform small cells with sparse, pale, basophilic cytoplasm, and bland, round to oval nuclei with granular chromatin and small chromocenters. They often are present in clusters or sometimes branching cords. A variable amount of amorphous, eosinophilic, stromal basement membrane-like material is present, usually adjacent to the cell clusters (Fig. 32-14B). Occasionally, the amorphous material is spherical and surrounded by small epithelial cells, mimicking adenoid cystic carcinoma (Fig. 32-14C,D). In some cases of tubular adenoma




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree