CHAPTER 7 SAFETY OF COMPLEMENTARY MEDICINES
Complementary medicines (CMs) are widely used by the public, who assume they are a safe, non-pharmaceutical option that can be used to prevent, treat and manage disease (MacLennan et al 2002). The perception that CMs are safe is largely based on the assumption that if something is ‘natural’ it is inherently safe, a view encouraged by many in the health food industry. Unfortunately the ‘natural’ argument is simplistic and not well thought out, because nature provides many examples of unsafe substances, such as the naturally occurring poisons hemlock, jimsonweed and oleander. There is also an assumption that possible side effects and toxicities will be listed on product labels and therefore, if the information is absent, the product must be well tolerated. In Australia, some warnings are required on labels; however, CMs are not accompanied by comprehensive consumer product information (CPI) in the same way as many pharmaceutical medicines; many CMs are self-selected without professional advice, which means that much-needed information is not delivered with the product (Jamison 2003, MacLennan et al 2002).
Importantly, people who use CMs tend to have poorer health than the general community (MacLennan et al 2006) and are not necessarily dissatisfied with their conventional care (Astin 1998), which raises the possibility of dual care by complementary and conventional medical practitioners. This situation is not necessarily dangerous and can produce significant benefits when well coordinated; however, if communication is poor, and complementary and conventional practitioners remain unaware of what the other has recommended, a potentially unsafe situation can arise. The prospect of interactions or adverse drug reactions leading to misdiagnosis, induction of withdrawal effects and misleading pathology test results are examples of unwanted outcomes when combined care is not coordinated.
A BRIEF HISTORY OF MEDICATION SAFETY
The potential for medical care to cause harm has been appreciated throughout history. In ancient times, knowledge of medicine, pharmacology and the healing arts developed through trial and error, with many adverse outcomes and deaths along the way. Although both practitioners and patients were aware that health could be compromised by the ‘cures’ used to alleviate disease, it was in ancient Greece that patient safety was formally acknowledged as the highest priority. The maxim primum non nocere (First, do no harm) is attributed by some historians to Galen (AD 131–201) and is still a basic tenet of modern medical practice (Ilan & Fowler 2005).
In the 16th century, Paracelsus (1493–1541) was one of the first physicians to believe that chemicals could cure and cause certain illnesses. He determined that specific chemicals were responsible for the toxicity of a plant or animal poison, and documented the body’s responses to those chemicals. Paracelsus then concluded that the body’s response was influenced by the dose received. He further discovered that a small dose of a substance may be harmless, or even beneficial, whereas a larger dose can be toxic. In essence, he started expounding the concept of a dose–response relationship. Paracelsus made an enormous contribution to medicine when he stated plainly, ‘What is there that is not poison? All things are poison and nothing (is) without poison. Solely the dose determines that a thing is not a poison’ (Watson 2005). As a result, he is sometimes referred to as the ‘Father of Toxicology’.
More recently, the traditional evidence base has been joined by a scientific evidence base, which provides additional information about pharmacological actions, clinical effects and safety; however, much still remains unknown.
Clinical note — History of poisons
The history of poisons dates back to the earliest times, when humans observed toxic effects in nature, most likely by chance. By 1500 BC, written records indicate that the poisons hemlock, opium and certain metals were used in warfare and in facilitating executions. Over time, poisons were used with greater sophistication; notable poisoning victims include Socrates, Cleopatra and Claudius. Today, the ‘science of poisons’ is known as toxicology. This field of learning investigates the chemical and physical properties of poisons and their physiological or behavioural effects on living organisms, and uses qualitative and quantitative methods for analysis and for the development of procedures to treat poisoning (Langman & Kapur 2006). The 20th century was marked by an advanced understanding of toxicology; DNA and various biochemicals that maintain cellular functions were discovered, so that today we are discovering the toxic effects on organs and cells at the molecular level.
This is particularly true regarding the safety of CMs in children and in women who are pregnant or lactating, and concerning drug interactions, which is a relatively new phenomenon. Just as Galen pronounced hundreds of years ago, ‘First, do no harm’ should remain the practitioner’s guide.
WHAT IS SAFETY?
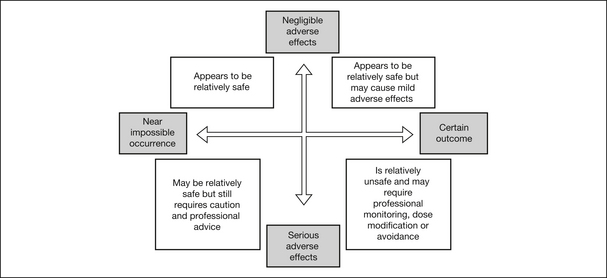
Safety is a complex issue that is determined by considering the interaction between ‘likelihood’ and ‘consequence’. These two variables will differ for each medicine and individual. The likelihood can be graded from ‘near impossible’ to ‘certainly likely’, and the severity of consequence can be graded from ‘negligible’ to ‘serious and life-threatening’, with many outcomes lying somewhere between these extremes (Fig 7.1).
With regard to medication safety, avoidance of an adverse drug reaction (ADR) is paramount. Several factors are associated with an increased likelihood of developing an ADR, such as advanced age and polypharmacy, but most ADRs occur in people who are prescribed treatment within the limits of accepted clinical practice (Burgess et al 2005).
BENEFITS, RISK AND HARM
Many different sources of risk are associated with therapeutic products:
The amount of safety literature published on pharmaceutical medicines is overwhelming. It has been estimated that 30% of the primary published literature about ADR appears in anecdotal reports and 35% as formal studies or randomised controlled trials (Aronson et al 2002). As regards the safety of complementary medicines, traditional evidence and theoretical reasoning are heavily relied upon to provide guidance because relatively little reliable information has been published in the peer-reviewed literature. This poses a challenge for practitioners when making a rational decision about the relative risks of treatment and is one of the great difficulties of CAM practice. For the public who are interested in using OTC products, it is just as difficult to find reliable and understandable information about their safety and efficacy.
ADVERSE DRUG REACTIONS (ADRs)
Type A reactions
Type A reactions are the most common form and are typically dose-related, predictable from the known pharmacology of the medicine, associated with high morbidity but low mortality, and potentially avoidable (Routledge et al 2004). People most at risk of a type
TABLE 7.1 Potential Risks Associated With the Use of Complementary Medicines
| Type of harm | Circumstances |
|---|---|
| Delay in diagnosis | When a patient has avoided or delayed seeking medical advice because they are self-treating with CMs. |
| Adverse effects | Increased risk of adverse reactions with inappropriate use of CM products or when patients self-select CM products without professional advice. |
| Drug interactions | Increased risk of drug interactions when patients: (a) self-select CM products without professional advice; (b) do not disclose use of CM products to their pharmacist or medical physician; (c) do not disclose use of pharmaceutical drugs to their CM practitioner. |
| Financial cost | If an expensive medicine or therapy is not providing benefits and a patient continues to use it, this presents an unnecessary financial burden. |
| Lost opportunity to treat | Failure to undertake a different treatment with proven benefits, when the current treatment is ineffective but is being used to the exclusion of others. |
| False hope of a cure | When cure is unlikely, the use of any medicine or therapy that is associated with false hope may delay important considerations, such as attending to ‘unfinished business’. |
A reaction are frail, older patients who are also likely to be receiving a combination of medicines and those with altered hepatic or renal function. There is now mounting evidence to indicate that some type A adverse reactions are due to genetic polymorphisms, which affect an individual’s drug clearance rate and therefore toxicological response. This may explain why certain individuals taking medicines in the recommended doses experience adverse reactions, whereas the majority of the population does not. Examples related to pharmaceutical medicine are bleeding with anticoagulants and hypoglycaemia with the use of insulin. An example for herbal medicine is licorice-associated hypertension, which is thought to be caused by increased renal sodium retention. The glycyrrhetinic acid in licorice inhibits renal 11-beta-hydroxysteroid dehydrogenase type 2 and, by that mechanism, increases the access of cortisol to the mineralocorticoid receptor that causes renal sodium retention and potassium loss. If continued for sufficient time, clinically significant changes in blood pressure and potassium status develop, which can be avoided by recommending that high-dose licorice herbal products not be used for longer than 2 weeks (Heilmann et al 1999). In recognition of this adverse effect, some manufacturers produce licorice products that do not contain glycyrrhetinic acid, so that they can be used more safely in the long term.
Table 7.2 gives some examples of known or suspected type A adverse reactions to herbs and natural supplements. For many herbal and natural medicines, there is insufficient reliable information about possible adverse reactions; where available, evidence from clinical trials, case reports and post-marketing surveillance systems are the main sources of information used in this book.
TABLE 7.2 Examples of Known or Suspected Type A Adverse Reactions to Herbs and Natural Supplements
| Herb or natural supplement | Adverse effect/s |
|---|---|
| Andrographis paniculata | Vomiting, anorexia and gastrointestinal discomfort |
| Creatine | Nausea, vomiting, cramping, dehydration, fluid retention |
| Trigonella foenum (fenugreek) | Diarrhoea, flatulence |
| Fish oils | Gastrointestinal discomfort, diarrhoea |
| Allium sativum (garlic) | Breath and body odour, nausea, dyspepsia, flatulence, diarrhoea, increased bleeding |
| Zingiber officinale (ginger) | Gastric irritation, dyspepsia |
| Camellia sinensis (green or black tea) | CNS stimulation |
| Gymnema sylvestre | Hypoglycaemia |
| Paullinia cupana (guarana) | CNS stimulation |
| Selenium | Nausea, vomiting, irritability, fatigue, nail changes |
Type B reactions
Type B reactions are idiosyncratic and uncommon, difficult to predict and not dose related. They tend to have higher morbidity and mortality than type A reactions and are often immunologically mediated (Myers & Cheras 2004). Other factors contributing to type B reactions are receptor or drug metabolism abnormalities and the unmasking of a biological deficiency (e.g. glucose-6-phosphate dehydrogenase deficiency) (Bryant et al 2003). They do not appear to relate to genetic polymorphisms.
An example of a type B reaction to a pharmaceutical drug is interstitial nephritis with the use of NSAIDs. With regard to CMs, Asteracaea dermatitis provides a good example of a type B hypersensitivity reaction — specifically, an allergic contact dermatitis caused by exposure to allergens from the Asteraceae family or the daisy group of plants and plant extracts. Some examples of common plants that belong to this family are arnica (Arnica montana), chamomile (Chamomilla recucita), marigold (Calendula officinalis), echinacea (Echinacea spp), tansy (Tanacetum vulgare), feverfew (Tanacetum parthenium) and yarrow (Achillea millefolium). The most important allergens in the Asteraceae family are the sesquiterpene lactones, which are present in the oleoresin fraction of the leaves, stems, flowers and possibly pollen (Gordon 1999). The condition is most frequently seen in middle-aged and elderly people; it typically starts in summer and disappears in the autumn or winter. The dermatitis manifests as eczema and can develop from exposure to airborne particles, direct topical application (such as cosmetics, perfumes, essential oils) or oral ingestion of allergenic components. The diagnosis of allergy can be difficult to establish, because there are few completely reliable laboratory tests and sometimes symptoms can mimic infectious disease symptoms. Table 7.3 gives examples of known or suspected type B adverse reactions.
TABLE 7.3 Examples of Known or Suspected Type B Adverse Reactions to Herbs and Natural Supplements
| Herb or natural supplement | Adverse effect/s |
|---|---|
| Andrographis paniculata | Urticaria |
| Aloe vera | Hypersensitivity and contact dermatitis |
| Chamomilla recutita | Asteraceae dermatitis |
| Echinacea spp | Asteraceae dermatitis and anaphylaxis |
| Tanacetum parthenium (feverfew) | Asteraceae dermatitis — lip swelling, mouth ulceration and soreness when the leaves are chewed |
| Zingiber officinale (ginger) | Contact dermatitis with topical use |
| Thymus vulgaris (thyme) | Contact dermatitis with topical use of the oil |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree