Robotic Surgery
Santiago Horgan
Michael F. Sedrak
Introduction
The human nature of the surgeon has been to gain the maximal access to the surgical area of interest while inflicting as minimal trauma to the patient as possible. As operative techniques and technologies have evolved, the ability to achieve that goal has advanced tremendously. Surgeons have gained the ability and technical expertise first to make smaller, more strategically located incisions for open surgical access, then to perform the same operation utilizing surgical telescopes and minimal access tools, then to the current state-of-the-art use of computer-aided technologies and robotics to further gain full access to what otherwise would have been difficult-to-approach surgical areas of interest while having nearly negligible secondary surgical trauma to the patient.
In truth of fact, as these technologies continue to demonstrate their immense utility as tools to significantly improve the surgeon’s ability to safely and superiorly treat disease, their use will ultimately become ubiquitous in the operating room much as the surgical telescopic devices have infiltrated surgical practice in recent years.
Hurdles to overcome include developing and improving the technologies and their limitations, improving physician training and expertise to maximize their abilities to safely and effectively use these tools, incorporating technologies training into the surgical education of trainees in the various disciplines, and of course managing the increased cost in a manner that will ultimately allow patients’ increased access to the best care available.
Robotic Platforms and Technology
Telemanipulation systems developed from modest beginnings two decades ago with two devices that used robotic technologies to automate camera positioning during telescopic procedures. The EndoAssist (Armstrong Health Care, High Wycombe, UK) used infrared sensors to detect the movements of the surgeon’s head in order to maneuver the camera. Later, under a NASA Small Business Innovation Research Contract from the Jet Propulsion Laboratory, Computer Motion Inc., Santa Barbara, CA, developed the Automated Endoscopic System for Optimal Positioning (AESOP)—a robotic arm system whose applications entered the operating room allowing the surgeon to control the surgical camera controlled by the robotic arm first with a foot-pedal interface, then later by voice commands as experience showed that this was the preferred method of controlling the arm.
Later, extending their technology, Computer Motion Inc. developed the Zeus Robotic Surgical System which was a master-slave robotic system that allowed the surgeon to control up to three surgical arms in addition to the camera system from a separate control console.
The Stanford Research Institute (SRI) developed a robotic surgical system with research funded from the National Institutes of Health and with interest from the Defense Advanced Research Projects Administration (DARPA). Ultimately, becoming the da Vinci Robotic Surgical System by Intuitive Surgical of Mountain View, CA, the da Vinci is also a master-slave robotic system, but with some very distinct advantages over its progenitors. The system includes a fully integrated three-dimensional (3-D), high-definition (HD) visualization system that allows a spectacular visualization of the magnified surgical topography. Further, the instruments have a fully articulating wrist that truly facilitates operating with such degree of freedom that allows full, natural motions in the tightest of surgical fields. Finally, the system has a fourth arm, thus mitigating the need for a trained assistant.
The U.S. Food and Drug Administration (FDA) has cleared the da Vinci Surgical System for use in urological surgical procedures, general laparoscopic surgical procedures, gynecologic laparoscopic surgical procedures, transoral otolaryngology surgical procedures restricted to benign and malignant tumors classified as T1 and T2, general thoracoscopic surgical procedures, and thoracoscopically assisted cardiotomy procedures. The system can also be employed with adjunctive mediastinotomy to perform coronary anastomosis during cardiac revascularization. The system is indicated for adult and pediatric use, and is recently approved for transoral otolaryngology surgical procedures. It is intended for use by trained physicians in an operating room environment in accordance with representative, specific procedures set forth in the Professional Instructions for Use.
Representative Uses: According to Intuitive Surgical, Inc., the da Vinci System has been successfully used in the following procedures, among others:
Urology
Radical prostatectomy, pyeloplasty, cystectomy, nephrectomy, ureteral reimplantation.
Gynecology
Hysterectomy, myomectomy, and sacrocolpopexy.
General Surgery
Cholecystectomy, Nissen fundoplication, Heller myotomy, gastric bypass, donor nephrectomy, adrenalectomy, splenectomy, and bowel resection.
Cardiac Surgery
Internal mammary artery mobilization and cardiac tissue ablation.
Mitral valve repair, endoscopic atrial septal defect closure.
Mammary to left anterior descending coronary artery anastomosis for cardiac revascularization with adjunctive mediastinotomy.
Otolaryngology
Oropharyngeal, laryngeal, and hypopharyngeal resections; floor of mouth and oral cavity resections.
Equipment and Setup Fundamentals
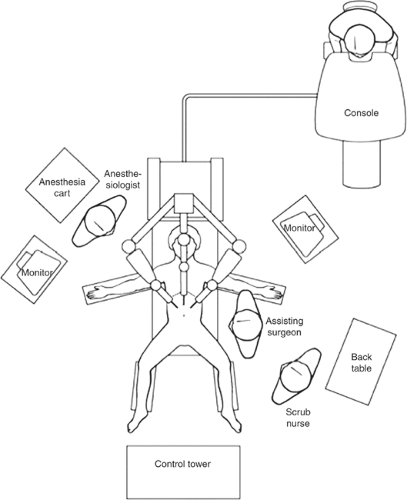
The da Vinci Surgical System has a surgeon console where the operator receives a binocular image from the surgical field and operates the robotic arms through a computerized system using master controls. In this system, depending on the model, three or four robotic arms are assembled on a movable single unit, with the central arm supporting two parallel high-definition cameras and the lateral arms serving as instrument arms. The image is acquired and delivered simultaneously to the console’s two monitors and is observed in a parallel binocular fashion by the surgeon’s eyes, allowing a magnified 3-D stereoscopic view with depth perception. Camera positioning is controlled by the operator at the console.
The instrument tips have seven degrees of freedom and wrist action controlled by the surgeon hand controls at the console that are greatly similar to the hand and wrist movement mechanics of open surgery, allowing more complex and delicate tasks than the standard laparoscopic instrumentation at
the tissue level. A clutching system allows readjustment of the surgeon hand positions at the console to maintain ergonomic control of the instruments within the surgical field of interest.
the tissue level. A clutching system allows readjustment of the surgeon hand positions at the console to maintain ergonomic control of the instruments within the surgical field of interest.
Principles of triangulation are generally employed with robotic system setup. Commonly, the patient cart, camera arm, and camera port are aligned in a straight line with the field of interest. The two working ports are placed on either side of the camera, allowing triangulation to the target field. A fourth port is placed in a manner conducive to the operation being performed and can be used for an assistant or for the fourth robotic arm. In situations where abdominal access is gained from a small area at the skin level, as in single incision surgery, the working ports can be reversed to allow a wide degree of freedom within the cavity, maintaining triangulation and minimizing equipment conflict.
Additionally, the cannulas are marked with a fulcrum point such that when the ports are inserted into the cavity of interest to the depth noted on the cannula, the full range of motion is optimized by the pre-measured fulcrum point into this remote center of focus as designed in the translational software of the robotic system to maximize the efficacy of the robotic arm movements with minimal torque onto the patient at the port entry site.
Finally, port and arm clutch maneuvers are used to dock the camera and instrument arms while maximizing the space between the instrument arms.
Robotic System Summary
Surgeon console: The surgeon operates while seated at a console using four pedals, a set of console switches, and two master controls. The movements of the surgeon’s fingers are transmitted by the master controls to the instrument located inside the patient. A 3-D image of the surgical field is obtained using a 12-mm scope, which contains two cameras that integrate images.
Control tower: This component contains a monitor, light sources, and cord attachments for the cameras.
Surgical arm cart: This component provides four robotic arms, three instrument arms, and one endoscope arm, which execute the surgeon’s commands.
General Surgery
Bariatric Surgery
Roux-en-Y gastric bypass has been described as being the gold-standard bariatric operation. It has been successfully performed laparoscopically for the past two decades, but is technically demanding. In addition to dealing with equipment torque on the abdominal wall due to patient girth, the most technically challenging step in the operation is completion of the gastrojejunal anastomosis. Multiple techniques have been described, including linear and circular stapling, as well as handsewn anastomosis (Fig. 1).
For the surgeon who prefers to handsew the gastrojejunostomy, use of computer-enhanced robotic equipment facilitates this step as the robotic arms are able to stabilize the telescope and instruments from the torque caused at the abdominal wall, thus maintaining instrument position and stability.
Utility of robotic assistance in adjustable gastric banding has been demonstrated in very high morbidly obese patients with BMI greater than 60 kg/m2 for the same reasons regarding abdominal wall girth as described earlier. However, the success of adjustable gastric banding in these patients compared to other operations such as sleeve gastrectomy or gastric bypass should be considered.
Robotic-assisted vertical sleeve gastrectomy will undoubtedly be demonstrated as an outstanding surgical option as long-term results of laparoscopic sleeve gastrectomy begin to confirm the long-term success of this surgical option to bariatric patients. Particularly useful is the stabilization of the camera and tools during handsewn staple line reinforcement in addition to the increased capacity of the general dissection.
Robotically Assisted Roux-En-Y Gastric Bypass Surgical Technique
The patient is placed in the low lithotomy position with the legs and arms open; a
beanbag is placed under the patient to support the steep reverse Trendelenburg position during the operation. A single dose of preoperative prophylactic antibiotics (first-generation cephalosporin) is given. Thigh-length antiembolic stockings and a sequential pneumatic compression device are placed on both lower extremities before induction of anesthesia. A single dose of 5,000 U subcutaneous heparin is given for prophylaxis against venous thrombosis. After general anesthesia is achieved, an NG tube is placed in the stomach and a Foley catheter is put in position.
beanbag is placed under the patient to support the steep reverse Trendelenburg position during the operation. A single dose of preoperative prophylactic antibiotics (first-generation cephalosporin) is given. Thigh-length antiembolic stockings and a sequential pneumatic compression device are placed on both lower extremities before induction of anesthesia. A single dose of 5,000 U subcutaneous heparin is given for prophylaxis against venous thrombosis. After general anesthesia is achieved, an NG tube is placed in the stomach and a Foley catheter is put in position.
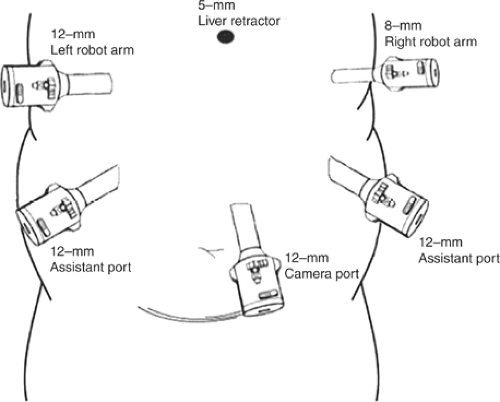
The trocar placement for robotically assisted Roux-en-Y gastric bypass is shown in Figure 2. The procedure starts by dividing the small bowel <50 cm below the angle of Treitz using a vascular stapler; the mesentery of the bowel is also divided using a vascular stapler. After creating a 150-cm limb, a jejunojejunal anastomosis is performed using 2 reloads of vascular staplers. The bowel opening is closed using an endo-needle holder with interrupted stitches of 3-0 silk. The defect between the mesentery is closed using a 3-0 silk suture.
At this time, the patient is placed in a reverse Trendelenburg position; the omentum is mobilized and sectioned using the harmonic scalpel. Next, beginning at the lesser curve (<5 cm from the gastroesophageal junction), the retrogastric tunnel is created using the harmonic scalpel. Several firings of the surgical stapler are performed to create a <30-cm3 gastric pouch; following completion, the distal portion of the ileum is brought up for creation of the gastrojejunostomy. At this time, the surgical arm patient-side cart robotic surgical system is positioned. To perform the gastrojejunal anastomosis, a Cadière forceps is attached to the right arm and a needle holder to the left arm. The posterior layer of the gastrojejunal anastomosis is performed with 3-0 silk. Then, using electrocautery, a 1.5-cm opening is created in both the jejunum and the gastric pouch; for the opening, the cautery is hooked to the left arm. Once the bowel and the stomach are opened, the handmade anastomosis using the robot is started. A running suture is placed to the right and left of the anastomosis using 3-0 absorbable suture. The anterior serosa–serosa layer of the gastrojejunal anastomosis is closed using 3-0 silk. Once the anastomosis is finished, the robotic surgical cart is removed from the patient’s side.
The NG tube is passed down into the gastric pouch. The distal limb of the ileum is clamped, and 60 mL of methylene blue is introduced to rule out the presence of leak. Patients are encouraged to ambulate on the same operative day. On postoperative day 1, patients undergo a Gastrografin swallow to evaluate the status of the gastrojejunal anastomosis. Following this, they start a clear liquid diet. On postoperative day 2, if no complications are experienced, they are discharged home.
Robotically Assisted Adjustable Gastric Banding
Surgical Technique
The patient is placed in the low lithotomy position with the legs and arms open. The surgeon operates between the patient’s legs, with the assistant at the patient’s left side. Prophylactic antibiotics (first-generation cephalosporin) as well as 5,000 U subcutaneous heparin are given to the patient during the anesthesia induction.
Four trocars are used. The first is a 10- to 12-mm bladeless trocar that is inserted under direct vision 15 to 20 cm from the xiphoid process using a 10-mm, 0-degree scope. Pneumoperitoneum is then achieved to 20 mm Hg. The rest of the trocars are placed under direct vision using a 30-degree scope. An 8-mm trocar (robotic arm) is placed immediately below the left rib cage in the mid clavicular line. An 18-mm trocar is then placed on the left flank at the same level as the camera. At this point, the patient is placed in the reverse Trendelenburg position, which allows for better visualization of the gastroesophageal junction. A 5-mm incision is made below the xyphoid process to facilitate introduction of a Nathanson liver retractor. The last 8-mm trocar (robotic arm) is placed <8 cm below the right rib cage, depending on the position of the liver edge.
The Cadière forceps are attached to the right arm and the harmonic scalpel to the left arm. The first step of the operation consists of detaching the phrenogastric ligament in order to expose the left crura. Then, the gastrohepatic ligament is opened. The caudate lobe of the liver, the inferior vena cava, and the right crura are subsequently exposed. Having identified these structures correctly, we create a retrogastric tunnel using blunt dissection. We start the dissection between the edge of the right crura and the posterior wall of the stomach, and it is continued until the articulated tip of the robotic instrument is visualized at the other side of the stomach, at the angle of His. At this time, and using the 18-mm trocar, the band is placed inside the abdomen. Following this, the tip of the tubing is placed between the jaws of the Cadière forceps, attached to the left arm, and the band is threaded around the stomach (Fig. 3). Since the tip of the instrument is articulated, there is no need to use the band passer. Then the tip of the tubing is inserted into the band buckle and locked. With the band closed and in position, a wrap is fashioned out of the stomach to secure the band in place. We place 3 (or 4 if necessary) nonabsorbable seromuscular sutures during the creation of this wrap (Fig. 4). The first is placed in the left lateral aspect of the gastric pouch, and two more are placed in the anterior aspect. Once the band is in position, the port is then secured using four polypropylene 2-0 sutures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree