Chapter Fifty-Three. Risks of infection and trauma in neonates
CHAPTER CONTENTS
Infections
Fetal and neonatal immunocompetence
Although not fully developed, the neonatal immune system is capable of mounting considerable defence against pathogens, removing worn out and damaged host cells, monitoring and destroying mutant cells. The term immune system subsumes both innate and specific immunity. Innate immune mechanisms are generally well developed at birth, always vigilant, rapidly activated and encoded in the individual’s genome. Components of innate immunity such as the neutrophils are responsible for immediate recognition, isolation and initiation of pathogen destruction. In contrast, the specific immune mechanisms are activated on exposure to specific pathogens (or vaccines), but these mechanisms require days or weeks for development and are invariably dependent on somatic gene rearrangements (Giroir 2003). The most important components of the specific immune system are the B and T lymphocytes. Both these naïve cell lineages must be exposed to and programmed by specific antigens. In response, the mature B lymphocytes are converted into antibody-producing plasma cells whereas the activated T cells synthesise and release cytokines such as interferons.
Bellanti et al (1999) define immaturity of the immune mechanisms as ‘the inability of the immune system to mount a genetically programmed immune response to antigens such as bacteria, viruses and mutant cells’. In fetuses, as in neonates, the functional immaturity of the immune system is generally attributed to the limited exposure of the B and T lymphocytes to antigens and pathogens capable of activating appropriate immunological mechanisms critical to the generation of competent, specific immune responses. Those wishing to review the immune system should read Chapter 29. Although most neonates have some specific immunity obtained by passive means from colostrum and breast milk, the relative immaturity of the acquired immune mechanisms can predispose some neonates to infection (Table 53.1).
| Possible barriers | Deficiencies in host defence mechanisms |
|---|---|
| Anatomical barriers | Skin abrasions sustained during delivery Invasive procedures: airway lavage, endotracheal intubation, umbilical artery catheterisation |
| Phagocytic cells | Small numbers of polymorphonuclear leucocytes Decreased polymorphonuclear cell activity Slow up-regulation in neutrophil production Poor transmission of neutrophils into the tissue |
| Complement mechanisms | Decreased levels of complement proteins in the blood, possibly due to immaturity of the liver |
| Cellular immunity | Possible defects in T-cell immunoregulation |
| Humoral immunity | Low levels of immunoglobulins IgA and IgM Low levels of IgG in premature infants Impaired antibody function Low levels of cytokines, e.g. interferon and tumour necrosis factor |
Sources of neonatal infection can be divided into three categories (Venkatesh 2006):
1. Transplacental acquisition.
2. Perinatal acquisition.
3. Postnatal infection.
Fetuses are vulnerable to pathogenic infections due to their relatively immature and inexperienced immune systems. Many of these conditions are covered in Chapter 14. Some of the prevalent fetal infections are:
• TORCH pathogens which cross the placenta relatively uninhibited (see Ch. 14).
• Any pathogens ascending the maternal reproductive tract, especially if the fetal membranes rupture early during the perinatal period.
• Other infections acquired during pregnancy from organisms such as Candida albicans, Neisseria gonorrhoeae, Chlamydia trachomatis, Listeria monocytogenes, herpes simplex, HIV infection (see Ch. 14).
Perinatal and postnatal infections
Neonates are colonised by commensals and pathogens during labour as well as after birth when they are exposed to new environments. Some of these pathogens are most likely to cause neonatal systemic and topical infections. Neonatal infections are difficult to recognise as early signs may be non-specific. The neonate may be lethargic, reluctant to feed, fretful, develop jaundice and have unexplained vomiting and unusual stool pattern with significant weight loss. Poor temperature control, manifesting as either pyrexia or more commonly hypothermia, accompanied by tachypnoea, apnoea, cyanosis and bradycardia must be carefully monitored. A neonate’s cold peripheries, mottled skin and grey or pale appearance are often late signs of systemic infection. Because in some instances such neonates collapse suddenly, differential diagnoses such as metabolic disturbances, respiratory or cardiovascular problems or intracranial bleeding cannot be ruled out.
Apparently healthy neonates who suddenly deteriorate must therefore be examined for evidence of infection. This should include microbiology screening and haemodynamic monitoring until a diagnosis is established. Early supportive and antimicrobial therapy may limit the severity of the infection, although delaying the diagnosis and deferring appropriate therapeutic interventions may contribute to the spread of pathogens and the development of septicaemia. In cases of doubt, neonates will undergo systematic screening, usually including obtaining nasal, throat, umbilical and skin swabs for microbiology cultures. A full blood profile, including white cell count, platelet count and blood cultures, is carried out. Cerebrospinal fluid obtained by lumbar puncture provides the means for screening neonates for evidence of meningitis and ventriculitis. Collection of urine and stool samples for microbial culture and pathogen sensitivity to antimicrobial drugs can provide an indication of the source of infection and influence the therapeutic management.
It is often expedient to administer broad-spectrum antimicrobials, frequently intravenously, until a diagnosis is confirmed and the pathogens and their sensitivity identified. Most neonatal units use specific combinations of antimicrobials such as amoxicillin and gentamicin, depending on which pathogens are present in that environment. Such neonates will also require supportive care to ensure that their body temperature is maintained, that they are well hydrated, adequately nourished and any risks of hypoglycaemia and acidosis are minimised (Giroir 2003). Fluid intake must be carefully managed as studies indicate that overhydration may lead to patent ductus arteriosus and necrotising enterocolitis (Bell & Acarregui 2001). Seriously ill neonates may require assisted mechanical ventilation.
Skin and surface infections
All neonatal skin lesions must be considered potentially abnormal, particularly if they are associated with staphylococcal infections, which may spread from the ear, nose, mouth and skin of a carer, another neonate or child. Due to the immaturity of the neonate’s immune system, simple lesions can rapidly lead to serious systemic infections. For instance, pyoderma, the appearance of small spots or pustules on the skin, and paronychia, a localised staphylococcal nail-bed infection, may spread rapidly in premature and ill neonates causing systemic infections. Both infections may require early antimicrobial therapy to minimise the risk of systemic infection.
Staphylococcal scalded skin syndrome is a serious skin infection caused by staphylococci entering the superficial soft tissue through a broken skin surface such as a scratch. Such infections are highly contagious and may lead to epidemics that result in closure of maternity units; therefore any blisters appearing on the neonate’s skin should be notified early to paediatricians as they may be due to exfoliative toxins produced by staphylococci (Hanakawa et al 2002). Generally these lesions appear on the head and the trunk and, if unattended, fill with pus, break and leave raw skin surfaces open to further infection. Extensive blisters may coalesce, giving a ‘scalded skin’ appearance. The neonate may become seriously ill with dehydration and may develop septicaemia. Affected neonates are cared for in an isolated environment. Supportive interventions include antibiotic therapy and rehydration. It is important to be aware of the prevalence of meticillin-resistant organisms that may not respond to the usual antibiotics.
Omphalitis or infection of the umbilicus can be serious because of a possible spread of common staphylococcal pathogens through the umbilical vein to the liver and kidneys (Fig. 53.1). Widespread erythema around the umbilicus or discharge of fluid or pus indicates local infection and must be treated with appropriate antibiotics and meticulous hygiene.
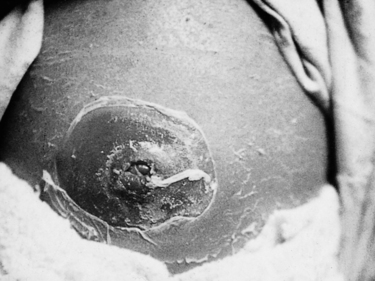 |
| Figure 53.1 Severe periumbilical infection. (From Kelnar C, Harvey D, Simpson C 1995, with permission.) |
Ophthalmia neonatorum means the presence of a purulent discharge from the neonate’s eye(s). Such infections may develop within 21 days of birth. Both eyes must be treated with appropriate antimicrobial therapy in order to minimise the risks of blindness. In severe cases, systemic antimicrobials are required. Although such severe eye infections are now rarely seen, pathogens such as the penicillin-resistant strains of Neisseria gonorrhoeae, staphylococci species, Escherichia coli and Chlamydia trachomatis can induce them. Care needs to be taken to ensure that neonates with such ocular infections are nursed with the affected eye in a downward position to ensure that the pathogens do not spread to the clean eye.
Serious infections
The most serious infections encountered in neonates are septicaemia, meningitis and pneumonia. In general, these infections present with non-specific signs such as thermoregulation problems, lethargy, apnoea, poor feeding and vomiting. Group B β-haemolytic Streptococcus aureus is frequently implicated in such neonatal sepsis (Oddie & Embleton 2002).
Septicaemia is a serious end-result of topical infection which, in most instances, is confirmed by blood culture. Besides the non-specific signs, abdominal distension, increased white blood cell count, hypotonia, unexplained metabolic acidosis and hypoglycaemia and hyperglycaemia may occur in premature and low-birth- weight neonates. Occasionally, the pathogens cause disseminated intravascular coagulopathy (DIC) in which case the septicaemia and coagulopathy require life-saving supportive and therapeutic interventions.
Meningitis is most likely to occur in premature neonates or those born after difficult pregnancies and deliveries. Group B β-haemolytic streptococcus and Escherichia coli are the most likely pathogens although Listeria monocytogenes, pneumococci, staphylococci and Candida albicans may be detected in cerebrospinal fluid cultures. The incidence of bacterial meningitis averages 0.4 per 1000 live births. Convulsions, bulging fontanelle, head retraction and hypothermia present in some neonates in the early stages of infection. Such symptoms must always be taken seriously and following investigative protocols managed proactively if mortality and the risks of long-term morbidity such as deafness, blindness and nerve palsies are to be minimised (Polin et al 2005).
Neonatal pneumonia may follow the inhalation of infected amniotic fluid or meconium causing respiratory distress within hours of birth. Conversely, aspiration pneumonia occurs in neonates who inhale milk or fluids given by nasogastric tube. This is most likely to occur in premature neonates whose swallowing and coughing reflexes are absent or weak. A range of pathogens can cause pneumonia with development of respiratory distress and cyanosis. All neonates require antimicrobial intervention and chest physiotherapy during the resolution phase of pneumonia. In milder forms of pneumonia, and where the neonate is relatively strong, being nursed in an incubator with humidified oxygen may be sufficient to ensure recovery. However, premature and very small neonates may require mechanical respiratory support. Nasogastric feeding is continued if possible, although neonates who manifest a degree of respiratory distress may benefit from intravenous hydration and nutrition.
Necrotising enterocolitis
Necrotising enterocolitis (NEC) is an acute inflammatory change affecting the small and large bowel in predominantly premature neonates (Anderson et al 2006, Berseth & Poenaru 2005). Although the aetiology remains unclear (Berseth & Poenaru 2005, Caplan & Jilling 2001), stress, infection, hypoxia, hypoglycaemia and inappropriate feeding may be contributing factors. Lucas & Cole (1990) showed that NEC is up to 10 times more common in exclusively formula-fed babies than those fed with breast milk alone, and three times more prevalent in those receiving both formula and breast milk feeds. The prevention of pathogenic presence by the early colonisation of the gut by lactobacilli may be crucial. If the neonate is not breastfed, factors such as IgA and lymphocytes from colostrum are absent, which allows invasion of the bowel wall, portal system and bowel lymphatic glands by bacteria such as Klebsiella, Clostridium and E. coli.
Partial- or full-thickness intestinal ischaemia usually involves the terminal ileum. The sloughing of the ischaemic mucosal layer (Berseth & Poenaru 2005) contributes to gas formation within the muscular layers and the formation of pneumatosis cystoides intestinalis




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


