15.1 INTRODUCTION TO RHABDOVIRUSES
The rhabdoviruses have minus-strand RNA genomes in the size range 11–15 kb. The name of these viruses is derived from the Greek rhabdos, which means a rod. The virions of some rhabdoviruses, especially those infecting plants, are in the shape of rods with rounded ends, while others, especially those infecting animals, are bullet shaped (Figure 15.1).
Figure 15.1 Negatively stained virions of vesicular stomatitis virus.
Source: Courtesy of Prof. Frederick A. Murphy, The University of Texas Medical Branch.

Rhabdoviruses are found in a wide range of hosts, including mammals, fish, plants, and insects, and many are important pathogens of animals and plants. The rhabdoviruses constitute the family Rhabdoviridae, which contains a number of genera, some of which are listed in Table 15.1.
Table 15.1 Examples of rhabdoviruses

Many rhabdoviruses have very wide host ranges and replicate in diverse types of host, especially the so-called “plant” rhabdoviruses, which replicate in their insect vectors as well as in their plant hosts (Chapter 4).
Before looking at rhabdovirus structure and replication, we consider two important rhabdoviruses, rabies virus and vesicular stomatitis virus (VSV).
15.2 SOME IMPORTANT RHABDOVIRUSES
15.2.1 Rabies virus
Rabies virus, like many rhabdoviruses, has an exceptionally wide host range. In the wild it has been found infecting many mammalian species, while in the laboratory it has been found that birds can be infected, as well as cell cultures from mammals, birds, reptiles, and insects.
Infection with rabies virus normally occurs as a result of virus in saliva gaining access to neurons through damaged skin. The infection spreads to other neurons in the central nervous system, then to cells in the salivary glands, where infectious virus is shed into the saliva (Figure 15.2).
Figure 15.2 Rabies virus infection of the animal body. After entering the body through damaged skin, a virion infects a neuron via the nerve endings and is transported to the cell body, where virus replication takes place. The infection spreads to other neurons and to salivary gland cells, which shed virions into the saliva.

Each year rabies kills large numbers of humans, dogs, cattle, and other animal species; precise numbers are not known, but for humans it is estimated that rabies causes more than 55 000 deaths annually. Most rabies infections of humans are acquired via bites from rabid dogs, though a few people have become infected after receiving an organ transplant from a rabies-infected individual. Rabies in humans is nearly always fatal, having a higher mortality than any other infectious disease.
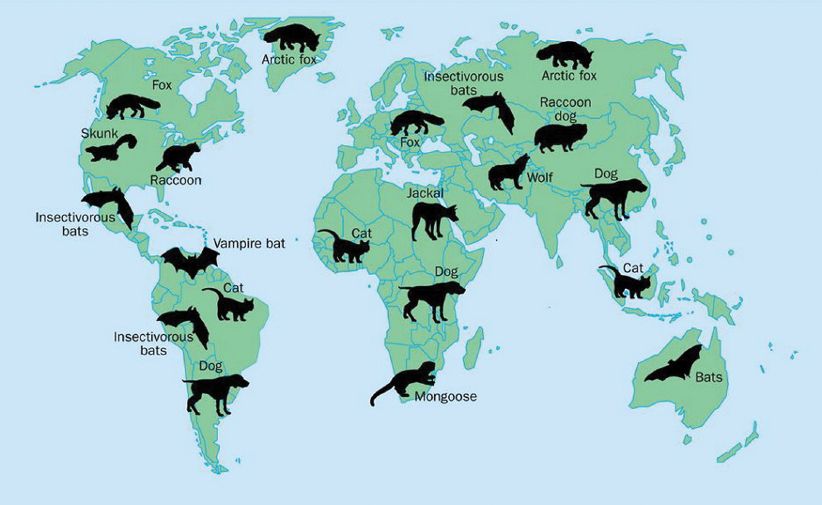
Rabies is endemic in wild animals in many parts of the world, often one animal species serving as the major reservoir (Figure 15.3). In Western Europe the major reservoir is the red fox.
Figure 15.3 Rabies virus reservoirs.
Source: Rupprecht et al. (2002) The Lancet Infectious Diseases, 2, 327. Reproduced by permission of Elsevier Limited and the authors.
Vaccines have been developed to provide protection to humans (e.g. veterinary surgeons), domestic animals (especially dogs), and wild animals (e.g. foxes) at risk from rabies virus infection. Rabies vaccines have been incorporated into food baits (Figure 15.4) attractive to wild mammals, and dropped from aircraft over fox-inhabited regions in Europe and coyote- and raccoon-inhabited regions in the US. The first vaccine to be used was an attenuated vaccine, but more recent vaccines have contained a recombinant vaccinia virus that expresses the rabies virus G protein. Vaccination of wild mammals has been very successful in bringing rabies under control in a number of countries.
Figure 15.4 Wild mammal bait containing rabies vaccine.
Source: Courtesy of Michael Roiland, Pinellas County, Florida, US.

Rabies is normally absent from the UK. In the past, this status was maintained through the requirement for a quarantine period for certain animal species, including dogs, on entry to the country. That policy has been largely replaced with a “pet passport scheme,” which involves giving rabies vaccine to animals prior to entry, and implanting an identifying microchip in each vaccinated animal.
Many viruses related to rabies virus have been found in bats around the world, and have been classified in the genus Lyssavirus along with the original rabies strains. Infection in the bat can have a number of possible outcomes: there may be subclinical infection, or there may be disease from which the bat may or may not recover. There are occasional cases of human rabies resulting from bites from infected bats. One such victim was David McRae, a licensed bat handler in Scotland, who died in 2002 after being bitten by an insectivorous bat.
15.2.2 Vesicular stomatitis virus
VSV causes disease in a variety of animals, including cattle, horses, sheep, and pigs, affected animals developing lesions on the feet and in the mouth similar to those in foot and mouth disease (Section 14.2.5). The disease can result in significant economic damage due to decreased milk and meat production, and the imposition of quarantines and trade barriers. Vesicular stomatitis is endemic in the tropics and there are epidemics in some temperate areas, but it has never been found in the UK.
VSV has a very wide host range. As well as infecting domestic livestock, there is evidence of infection in wild animals including bats, deer, and monkeys. This evidence is the presence in these animals of neutralizing antibodies to the virus. VSV has been isolated from a number of insect species, including mosquitoes, sand flies, and black flies. Its natural cycle is unknown, but it is possible that it is transmitted between mammals by one or more of these types of insect.
In the laboratory, VSV can replicate in cell cultures derived from mammals, birds, fish, insects, and nematode worms. Much of our understanding of rhabdovirus structure and replication comes from studies with VSV, which is much safer than rabies virus to work with. Three species of VSV are recognized.
15.3 THE RHABDOVIRUS VIRION AND GENOME ORGANIZATION
The rhabdovirus virion is an enveloped, rod- or bullet-shaped structure containing five protein species (Figures 15.1 and 15.5).
Figure 15.5 Rhabdovirus virion and genome organization. Between each of the genes for the structural proteins is a short intergenic sequence. At the ends of the genome are non-coding sequences: a “leader” at the 3′ end and a “trailer” at the 5′ end.

The nucleoprotein (N) coats the RNA at the rate of one protein molecule to nine nucleotides, forming a nucleocapsid with helical symmetry. Associated with this ribonucleoprotein are the minor virion proteins P (phosphoprotein) and L (large). The L protein is well named, its gene taking up about half of the genome (Figure 15.5). Its large size points to the fact that it is a multifunctional protein, as will be described later. The helical ribonucleoprotein is coated with a helical layer of M (matrix) protein, forming a layer between the nucleocapsid and the envelope. Trimers of G (glycoprotein) form spikes that protrude from the envelope.
The genomes of all rhabdoviruses encode these five proteins, and many rhabdoviruses encode one or more additional proteins.
15.4 RHABDOVIRUS REPLICATION
15.4.1 Attachment and entry
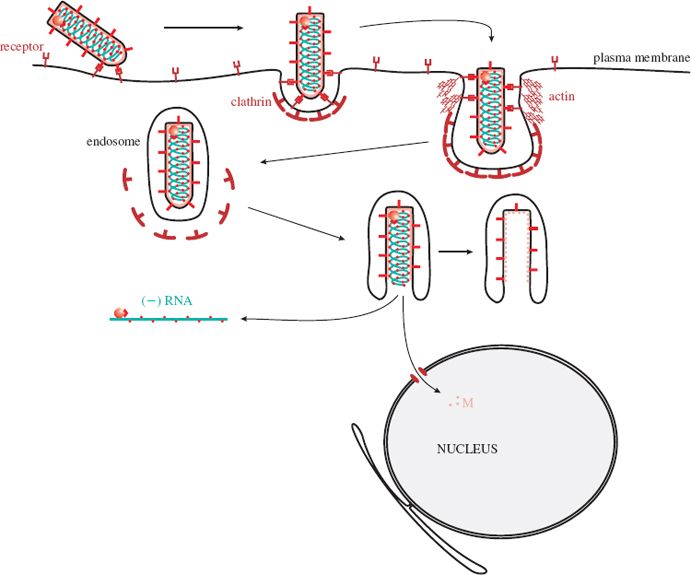
A rhabdovirus virion attaches to receptors at the cell surface and is then taken into the cell by endocytosis (Figure 15.6). At first, the invagination of the plasma membrane forms a clathrin-coated pit, but as the pit deepens the coating of the plasma membrane is formed from the protein actin, rather than clathrin. The G protein spikes of the virus envelope are involved in attachment to cell receptors and in fusion of the virion and endosome membranes. This fusion takes place after G molecules have undergone a conformational change induced by a drop in pH within the endosome. The nucleocapsid is released into the cytoplasm after the membranes of the virion and the endosome have fused. Most M protein remains associated with the endosome, but some is released and transported to the nucleus, where it acts to shut down expression of cell genes (Section 15.4.6).
Figure 15.6 Attachment and entry of a rhabdovirus. After endocytosis the membranes of the virion and the endosome fuse, releasing the nucleocapsid into the cytoplasm. Some M protein is transported to the nucleus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


