INTRODUCTION
The respiratory system distributes air to the alveoli, where gas exchange—the addition of oxygen (O2) and the removal of carbon dioxide (CO2) from pulmonary capillary blood—takes place. Certain specialized structures within this system play a vital role in preparing air for use by the body. The nose, for example, contains vestibular hairs that filter the air and an extensive vascular network that warms it. The nose also contains a layer of goblet cells and a moist mucosal surface; water vapor enters the airstream from this mucosal surface to saturate inspired air as it’s warmed in the upper airways. Ciliated mucosa in the posterior portion of the nose and nasopharynx as well as major portions of the tracheobronchial tree propel particles deposited by impaction or gravity to the oropharynx, where the particles are swallowed.
EXTERNAL RESPIRATION
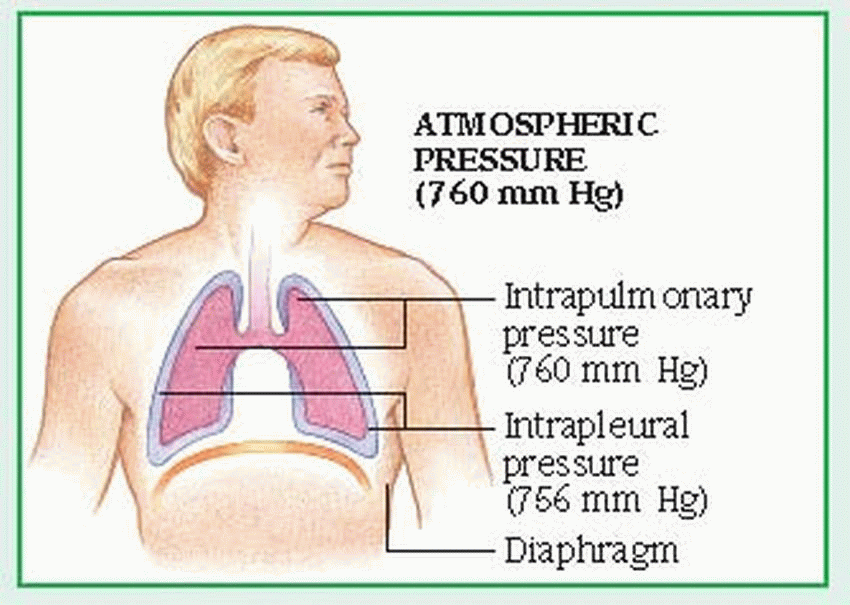
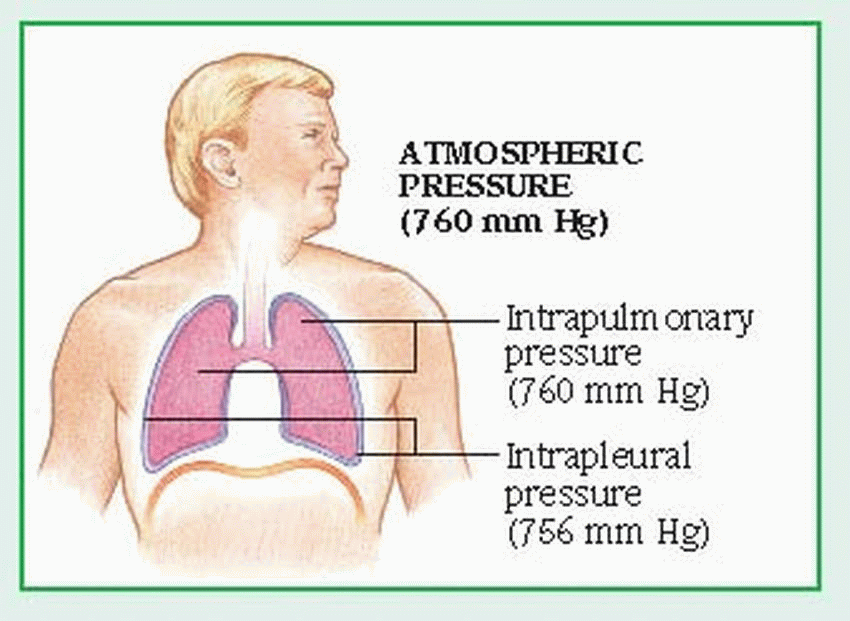
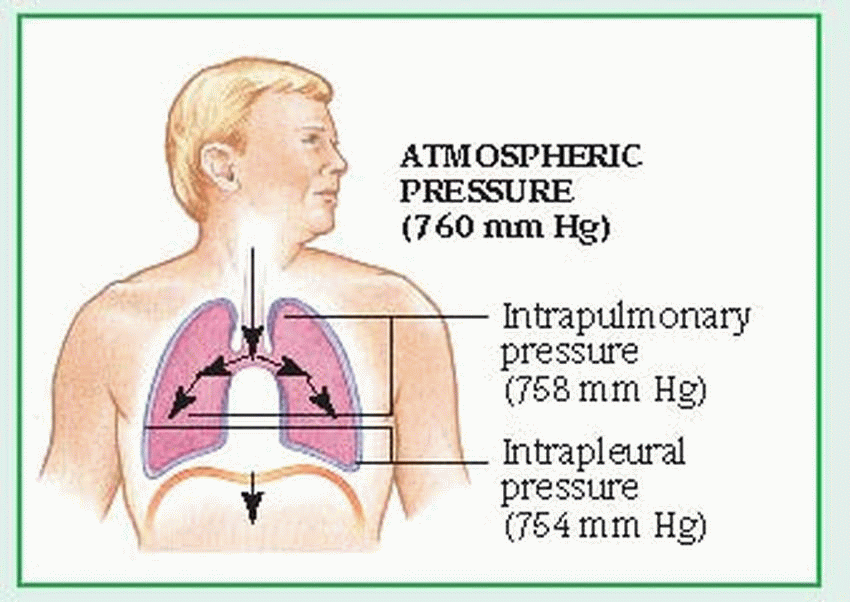
The external component of respiration— ventilation or breathing—delivers inspired air to the lower respiratory tract and alveoli. Contraction and relaxation of the respiratory muscles move air into and out of the lungs. Ventilation begins with the contraction of the inspiratory muscles: the diaphragm (the major muscle of respiration) descends, while external intercostal muscles move the rib cage upward and outward.
Air then enters the lungs in response to the pressure gradient between the atmosphere and the lungs. The lungs adhere to the chest wall and diaphragm because of the vacuum created within the pleural space. As the thorax expands, negative pressure is created in the intrapleural space, causing the lungs to also expand and draw in the warmed, humidified air. The accessory muscles of inspiration, which include the scalene and sternocleidomastoid muscles, raise the clavicles, upper ribs, and sternum. The accessory muscles aren’t used in normal inspiration but may be used in some pathologic conditions.
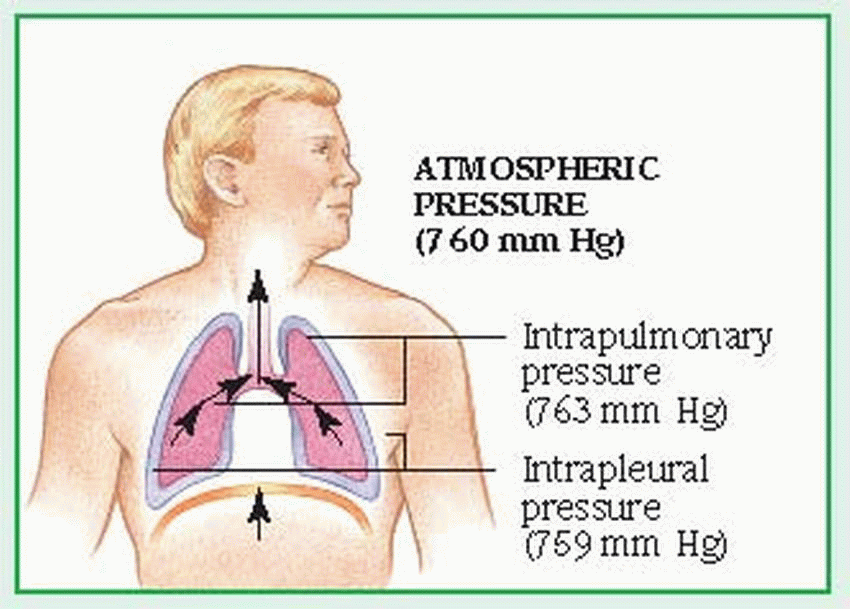
Normal expiration is passive; the inspiratory muscles cease to contract, the diaphragm rises, and the elastic recoil of the lungs causes the lungs to contract. These actions raise the pressure within the lungs above atmospheric pressure, moving air from the lungs to the atmosphere. Active expiration causes pleural pressure to become less negative. (See
Mechanics of ventilation,
page 90.)
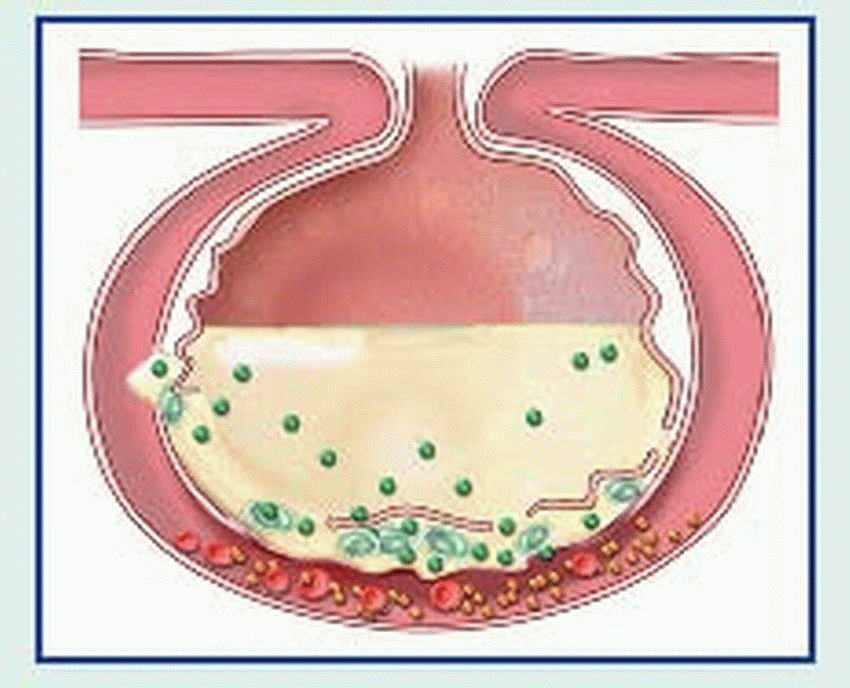
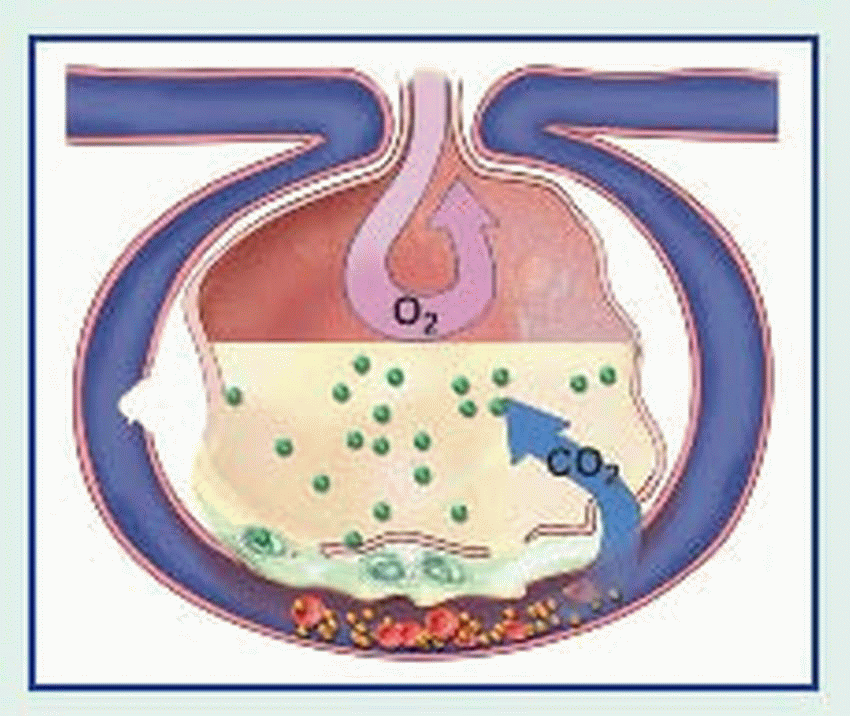
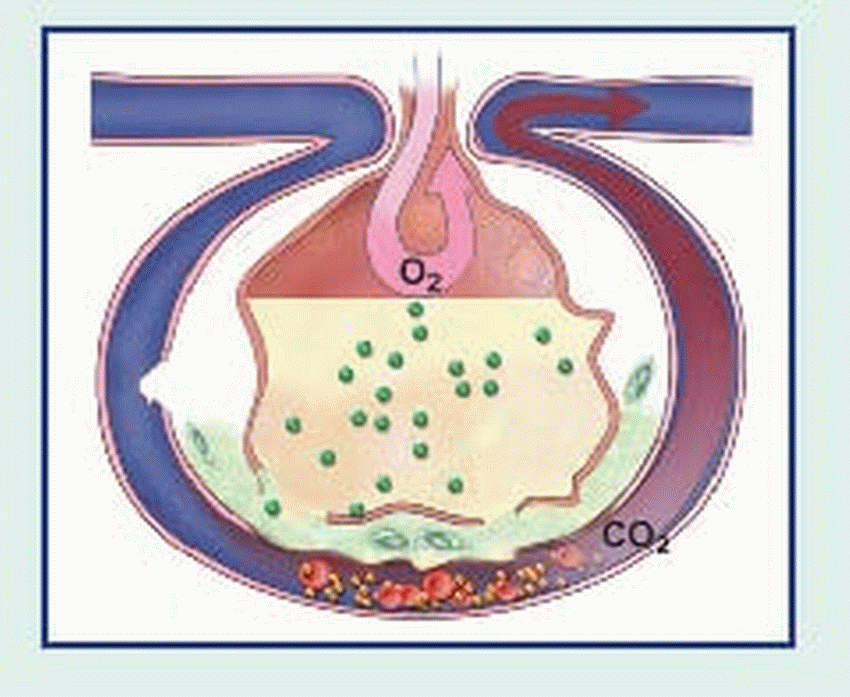
An adult lung contains an estimated 300 million alveoli; each alveolus is supplied by many capillaries. To reach the capillary lumen, O2 must cross the alveolocapillary membrane, which consists of an alveolar epithelial cell, a thin interstitial space, the capillary basement membrane, and the capillary endothelial cell membrane. The O2 tension of air entering the respiratory tract is approximately 150 mm Hg. In the alveoli, inspired air mixes with CO2 and water vapor, lowering the O2 pressure to approximately 100 mm Hg. Because alveolar partial pressure of O2 is higher than that present in mixed venous blood entering the pulmonary capillaries (approximately 40 mm Hg), O2 diffuses across the alveolocapillary membrane into the blood.
O2 AND CO2 TRANSPORT AND INTERNAL RESPIRATION
Circulating blood delivers O2 to the cells of the body for metabolism and transports metabolic wastes and CO2 from the tissues back to the lungs. When oxygenated arterial blood reaches tissue capillaries, O2 diffuses from the blood into the cells because of the O2 tension gradient. The amount of O2 available is determined by the concentration of hemoglobin (Hb; the principal carrier of O2), the percentage of O2 saturation of the Hb, regional blood flow, arterial O2 content, and cardiac output.
Internal (cellular) respiration occurs as a part of cellular metabolism, which can take place with O2 (aerobic) or without O2 (anaerobic). The most efficient method for providing fuel (highenergy compounds such as adenosine triphosphate [ATP]) for cellular reactions is aerobic metabolism, which produces CO2 and water in addition to ATP. Anaerobic metabolism is less efficient because a cell produces only a limited amount of ATP and yields lactic acid as well as CO2 as a metabolic by-product.
Because circulation is continuous, CO2 doesn’t normally accumulate in tissues. CO2 produced during cellular respiration diffuses from tissues into regional capillaries and is transported by systemic venous circulation. When CO2 reaches the alveolar capillaries, it diffuses into the alveoli, where the partial pressure of CO2 is lower; CO2 is removed from the alveoli during exhalation.
MECHANISMS OF CONTROL
The central nervous system’s (CNS’s) control of respiration lies in the respiratory center, located in the lateral medulla oblongata of the brain stem. Impulses travel down the phrenic nerves to the diaphragm, and down the intercostal nerves to the intercostal muscles, where the impulses change the rate and depth of respiration. The
inspiratory and expiratory centers, located in the posterior medulla, establish the involuntary rhythm of the breathing pattern.
Apneustic and pneumotaxic centers in the pons influence the pattern of breathing. Stimulation of the lower pontine apneustic center (e.g., by trauma, tumor, or stroke) produces forceful inspiratory gasps alternating with weak expiration. The apneustic center continually excites the medullary inspiratory center and thus facilitates inspiration. Signals from the pneumotaxic center as well as afferent impulses from the vagus nerve inhibit the apneustic center and “turn off” inspiration. The apneustic pattern doesn’t occur if the vagus nerves are intact.
Partial pressure of arterial oxygen (Pao2), pH, and pH of cerebrospinal fluid (CSF) influence output from the respiratory center. When CO2 enters the CSF, the pH of CSF falls, stimulating central chemoreceptors to increase ventilation.
The respiratory center also receives information from peripheral chemoreceptors in the carotid and aortic bodies. These chemoreceptors respond primarily to decreased Pao
2 but also to decreased pH. The peripheral
chemoreceptors have little control over respirations until the Pao
2 is less than 60 mm Hg.
During exercise, stretch receptors in lung tissue and the diaphragm prevent overexpansion of the lungs. During swallowing, the cortex can interrupt automatic control of ventilation. During sleep, respiratory drive may fluctuate, producing hypoventilation and periods of apnea. External sensations, drugs, chronic hypercapnia, and changes in body temperature can also alter the respiratory pattern.
DIAGNOSTIC TESTS
Diagnostic tests evaluate physiologic characteristics and pathologic states within the respiratory tract.
Noninvasive tests include:
Chest X-ray shows such conditions as atelectasis, pleural effusion, infiltrates, pneumothorax, lesions, mediastinal shifts, pulmonary edema, and chronic obstructive pulmonary disease (COPD).
Computed tomography (or CT) scan provides a three-dimensional picture that’s 100 times more sensitive than a chest X-ray.
Magnetic resonance imaging (or MRI) identifies obstructed arteries and tissue perfusion, but movement of the heart and lungs reduces the image’s clarity.
Sputum specimen analysis assesses sputum quantity, color, viscosity, and odor; microbiological stains and culture of sputum can identify infectious organisms; and cytologic preparations can detect respiratory tract neoplasms. Sensitivity tests determine antibiotic sensitivity and resistance.
Pulmonary function tests (or PFT) measure lung volume, flow rates, and compliance. Normal values, individualized by body stature, ethnicity, and age, are reported in percentage of the normal predicted value. Static measurements are volume measurements that include tidal volume, volume of air contained in a normal breath; functional residual capacity, volume of air remaining in the lungs after normal expiration; vital capacity, volume of air that can be exhaled after maximal inspiration; residual volume, air remaining in the lungs after maximal expiration; and total lung capacity (TLC), volume of air in the lungs after maximal inspiration. Dynamic measurements characterize the movement of air into and out of the lungs and show changes in lung mechanics. They include measurement of forced expiratory volume in 1 second, maximum volume of air that can be expired in 1 second from total lung capacity; maximal voluntary ventilation, volume of air that can be expired in 1 minute with the patient’s maximum voluntary effort; and forced vital capacity, maximal volume of air that the patient can exhale from TLC. (Peak flow rate, which can be obtained at the bedside, is also a dynamic measurement of pulmonary function.)
Methadholine challenge is one method of assessing airway responsiveness and is used to determine a diagnosis of asthma.
Exercise stress test evaluates the ability to transport O2 and remove CO2 with increasing metabolic demands.
Polysomnography can diagnose sleep disorders.
Lung scan (ventilation-perfusion or scintiphotography scan) demonstrates ventilation and perfusion patterns. It’s used primarily to evaluate pulmonary embolus.
Arterial blood gas (ABG) analysis assesses gas exchange. Decreased Pao2 may indicate hypoventilation, ventilation-perfusion mismatch, or shunting of blood away from gas exchange sites. Increased partial pressure of arterial carbon dioxide (Paco2) reflects marked ventilation-perfusion mismatch or hypoventilation; decreased Paco2 reflects increased alveolar ventilation. Changes in pH may reflect metabolic or respiratory dysfunction.
Pulse oximetry is a noninvasive assessment of arterial oxygen saturation.
Capnography may be used either transcutaneously or in ventilator circuit to determine Paco2 trends.
Invasive tests include:
Bronchoscopy permits direct visualization of the trachea and mainstem, lobar, segmental, and subsegmental bronchi. It may be used to localize the site of lung hemorrhage, visualize masses in these airways, and collect respiratory tract secretions. Brush biopsy may be used to obtain specimens from the lungs for microbiological stains, culture, and cytology. Lesion biopsies may be performed by using small forceps under direct visualization (when present in the proximal airways) or with the aid of fluoroscopy (when present distal to regions of direct visualization). Bronchoscopy can also be used to clear secretions and remove foreign bodies.
Thoracentesis permits removal of pleural fluid for analysis.
Pleural biopsy obtains pleural tissue for histologic examination and culture.
Pulmonary artery angiography, the injection of dye into the pulmonary artery, can locate pulmonary embolism. This is considered
the gold standard for diagnosing pulmonary emboli.
Positron emission tomography (or PET) scan uses a short-life radionuclide. Increased uptake of the substance is seen in malignant cells.
ASSESSMENT
Assessment of the respiratory system begins with a thorough patient history. Ask the patient to describe his respiratory problem. How long has he had it? How long does each attack last? Does one attack differ from another? Does any activity in particular bring on an attack or make it worse? What relieves the symptoms? Always ask whether the patient was or is a smoker, what and how often he smoked or smokes, and how long he smoked or has been smoking. Record this information in pack years—the number of packs of cigarettes per day multiplied by the number of smoking years. Remember to ask about the patient’s occupation, hobbies, and travel; some of these activities may involve exposure to toxic or allergenic substances.
If the patient has dyspnea, ask if it occurs during activity or at rest. What position is the patient in when dyspnea occurs? How far can he walk? How many flights of stairs can he climb? Has his exercise tolerance been decreasing? Can he relate dyspnea to allergies or environmental conditions? Does it occur only at night, during sleep? If the patient has a cough, ask about its severity, persistence, and duration; ask if it produces sputum and, if so, how much and what kind. Have the patient’s cough habits and character of sputum changed recently?
PHYSICAL EXAMINATION
Use inspection skills to check for clues to respiratory disease, beginning with the patient’s general appearance. If he’s frail or cachectic, he may have
a chronic disease that has impaired his appetite. If he’s diaphoretic, restless, or irritable or protective of a painful body part, he may be in acute distress. Also, look for behavior changes that may indicate hypoxemia or hypercapnia. Confusion, lethargy, bizarre behavior, or quiet sleep from which he can’t be aroused may point to hypercapnia. Watch for marked cyanosis, indicated by bluish or ashen skin (usually best seen on the lips, tongue, earlobes, and nail beds), which may be due to hypoxemia or poor tissue perfusion.
Assess chest shape and symmetry at rest and during ventilation. Increased anteroposterior diameter (“barrel chest”) characterizes emphysema. Kyphoscoliosis also alters chest configuration, which in turn restricts breathing. Assess respiratory excursion and observe for accessory muscle use during breathing. The use of upper chest and neck muscles is normal only during physical stress.
Observe the rate and pattern of breathing because certain disorders produce characteristic changes in breathing patterns. For example, an acute respiratory disorder can produce tachypnea (rapid, shallow breathing) or hyperpnea (increased rate and depth of breathing); intracranial lesions can produce Cheyne-Stokes and Biot’s respirations; increased intracranial pressure can result in central hyperventilation and apneustic or ataxic breathing; metabolic disorders can cause Kussmaul’s respirations; and airway obstruction can lead to prolonged forceful expiration and pursed-lip breathing.
Also observe posture and carriage. A patient with COPD, for example, usually supports rib cage movement by placing his arms on the sides of a chair to increase expansion and leans forward during exhalation to help expel air.
Palpation of the chest wall detects areas of tenderness, masses, changes in fremitus (palpable vocal vibrations), or crepitus (air in subcutaneous tissues). To assess chest excursion and symmetry, place your hands in a horizontal position, bilaterally on the posterior chest, with your thumbs pressed lightly against the spine, creating folds in the skin. As the patient takes a deep breath, your thumbs should move quickly and equally away from the spine. Repeat this with your hands placed anteriorly, at the costal margins (lower lobes) and clavicles (apices). Unequal movement indicates differences in expansion, seen in atelectasis, diaphragm or chest wall muscle disease, or splinting due to pain.
Auscultation normally detects soft, vesicular breath sounds throughout most of the lung fields. Absent or adventitious breath sounds may indicate fluid in small airways or interstitial lung disease (crackles), secretions in moderate and large airways (rhonchi), and airflow obstruction (wheezes).
SPECIAL RESPIRATORY CARE
The hospitalized patient with respiratory disease may require an artificial upper airway, chest tubes, chest physiotherapy, and supervision of mechanical ventilation. In cardiopulmonary arrest, establishing an airway always takes precedence. In a patient with this condition, airway obstruction usually results when the tongue slides back and blocks the posterior pharynx. The head-tilt method or, in suspected or confirmed cervical fracture or arthritis, the jaw-thrust maneuver can immediately push the tongue forward, relieving such obstruction. Endotracheal (ET) intubation and, sometimes, a tracheotomy may be necessary.
CHEST TUBES
An important procedure in patients with respiratory disease is chest tube drainage, which removes air or fluid from the pleural space. This allows the collapsed lung to reexpand to fill the evacuated pleural space. Chest drainage also allows removal of pleural fluid for culture. Chest tubes are commonly used after thoracic surgery, penetrating chest wounds, pleural effusion, and empyema. They’re also used for evacuation of pneumothorax, hydrothorax, or hemothorax. Sometimes chest tubes are used to instill sclerosing drugs into the pleural space to prevent recurrent malignant pleural effusions.
Commonly, the chest tube is placed in the sixth or seventh intercostal space, in the axillary region. Occasionally, in pneumothorax, the tube is placed in the second or third intercostal space, in the midclavicular region.
Follow these guidelines when caring for a patient with a chest tube:
Monitor changes in suction pressure.
Make sure that all connections in the system are tight and secured with tape.
Never clamp the chest tube unless checking for air leaks or changing the drainage system.
Record the amount, color, and consistency of drainage. Watch for signs of shock, such as tachycardia and hypotension, if drainage is excessive.
Encourage the patient to cough and breathe deeply every hour to enhance lung expansion.
Additionally, if a water seal-wet suction system is in place:
Check for fluctuation in the water-seal chamber as the patient breathes. Normal fluctuations of 2” to 4” (about 5 to 10 cm) reflect pressure changes in the pleural space during respiration.
Watch for intermittent bubbling in the waterseal chamber. This bubbling occurs normally when the system is removing air from the pleural cavity. Absence of bubbling indicates that the pleural space has sealed.
Check the water level in the suction-control chamber. If necessary, add sterile water to bring the level to the ordered level.
Check for gentle bubbling in the suction-control chamber, which indicates that the proper suction level has been reached.
If a dry-suction system is in place, check that the rotary dry-suction control dial is turned to the ordered suction mark and verify that the appropriate indicator is present, indicating that the desired amount of suction is applied.
VENTILATOR METHODS
Mechanical ventilators are typically used for CNS problems, hypoxemia, or failure of the normal bellows action provided by the diaphragm and rib cage. Positive-pressure ventilators cause inspiration while increasing tidal volume (VT). The inspiratory cycles of these ventilators may vary in volume, pressure, time, or frequency. For example, a volume-cycled ventilator—the type most commonly used—delivers a preset volume of air each time, regardless of the amount of lung resistance. A pressure-cycled ventilator generates flow until the machine reaches a preset pressure regardless of the volume delivered or the time required to achieve the pressure. A time-cycled ventilator generates flow for a preset amount of time. A highfrequency ventilator uses high respiratory rates and low VT to maintain alveolar ventilation. Positive end-expiratory pressure (PEEP) is used to retain a certain amount of pressure in the lungs at the end of expiration. By keeping small airways and alveoli open with this method, functional residual capacity is increased and oxygenation is improved.
Implement strategies to prevent ventilatorassociated pneumonia and plan to remove the patient from ventilator support as soon as the cause of respiratory failure has resolved (see Preventing ventilator-associated pneumonia). Several weaning methods are used. The patient may be taken off the ventilator and supplied with a T-piece (ET tube O2 adapter) that provides O2 and humidification. The patient then breathes spontaneously without the ventilator for gradually increasing periods.
With intermittent mandatory ventilation, the ventilator provides a specific number of breaths, and the patient is able to breathe spontaneously between ventilator breaths. The frequency of ventilator breaths is gradually decreased until the patient can breathe on his own. Pressure support ventilation, in which the patient receives a preset pressure boost with each spontaneous breath, has proved effective. Vital signs, ABG levels, physical findings, and subjective symptoms should be monitored periodically during weaning to assess respiratory status.
Chest physiotherapy
In respiratory conditions marked by excessive accumulation of secretions in the lungs, chest physiotherapy may enhance removal of secretions. Chest physiotherapy includes chest assessment, effective breathing and coughing exercises, postural drainage, percussion, vibration, and evaluation of the therapy’s effectiveness. Before initiating treatment, review X-rays and physical assessment findings to locate areas of secretions.
Deep breathing maintains diaphragm use, increases negative intrathoracic pressure, and promotes venous return; it’s especially important when pain or dressings restrict chest movement. An incentive spirometer can provide positive visual reinforcement to promote deep breathing.
Pursed-lip breathing is used primarily in obstructive disease to slow expiration and prevent small airway collapse. Such breathing slows air through smaller bronchi, maintaining positive pressure and preventing collapse of small airways and resultant air trapping.
Segmental breathing or lateral costal breathing is used after lung resection and for localized disorders. Place your hand over the lung area on the affected side. Instruct the patient to try to push that portion of his chest against your hand on deep inspiration. You should be able to feel this with your hand.
Coughing that’s controlled and staged gradually increases intrathoracic pressure, reducing pain and bronchospasm of explosive coughing. When wound pain prevents effective coughing, splint the wound with a pillow, towel, or your hand during coughing exercises.
Postural drainage uses gravity to drain secretions into larger airways, where they can
be expectorated. This technique is used in the patient with copious or tenacious secretions. Before performing postural drainage, auscultate the patient’s chest and review chest X-rays to determine the best position for maximum drainage. To prevent vomiting, schedule postural drainage at least 1 hour after meals.
Percussion moves air against the chest wall, enhancing the effectiveness of postural drainage by loosening lung secretions. Percussion is contraindicated in severe pain, extreme obesity, cancer that has metastasized to the ribs, crushing chest injuries, bleeding disorders, spontaneous pneumothorax, spinal compression fractures, and in patients with temporary pacemakers.
Vibration can be used with percussion or alone when percussion is contraindicated.
PEEP therapy maintains positive pressure in airways, preventing small airway collapse.
Before and after chest physiotherapy, auscultate the patient’s lung fields and assess for sputum production to evaluate the effectiveness of therapy.
CONGENITAL AND PEDIATRIC DISORDERS
Respiratory distress syndrome
Respiratory distress syndrome (RDS), also called hyaline membrane disease, is the most common cause of neonatal mortality. In the United States alone, it kills 40,000 neonates every year. RDS occurs in premature neonates and, if untreated, is fatal within 72 hours of birth in up to 14% of neonates weighing less than 5½ lb (2.5 kg). Aggressive management using mechanical ventilation can improve the prognosis, but some surviving neonates may develop some degree of bronchopulmonary dysplasia.
CAUSES AND INCIDENCE
Although airways and alveoli of a neonate’s respiratory system are present by 27 weeks’
gestation, the intercostal muscles are weak and the alveolar capillary system is immature. The preterm neonate with RDS develops widespread alveolar collapse due to a lack of surfactant, a lipoprotein present in alveoli and respiratory bronchioles. Surfactant lowers surface tension and helps prevent alveolar collapse. This surfactant deficiency results in widespread atelectasis, which leads to inadequate alveolar ventilation with shunting of blood through collapsed areas of lung, causing hypoxemia and acidosis.
RDS occurs almost exclusively in neonates born before 37 weeks’ gestation (in 60% of those born before the 28th week). The incidence is greatest in those with birth weights of 1,000 to 1,500 g. Infants of diabetic mothers, those born by cesarean delivery, second-born twins, infants with perinatal asphyxia, and those delivered suddenly after antepartum hemorrhage are more commonly affected.
SIGNS AND SYMPTOMS
Although a neonate with RDS may breathe normally at first, he usually develops rapid, shallow respirations within minutes or hours of birth, with intercostal, subcostal, or sternal retractions; nasal flaring; and audible expiratory grunting. This grunting is a natural compensatory mechanism designed to produce positive end-expiratory pressure (PEEP) and prevent further alveolar collapse.
Severe disease is marked by apnea, bradycardia, and cyanosis (from hypoxemia, left-toright shunting through the foramen ovale, or right-to-left intrapulmonary shunting through atelectatic regions of the lung). Other clinical features include pallor, frothy sputum, and low body temperature as a result of an immature nervous system and the absence of subcutaneous fat.
Sudden infant death syndrome
A medical mystery of early infancy, sudden infant death syndrome (SIDS), also called crib death, is the unexpected, sudden death of an infant or child younger than age 1 year. Reasons for the death remain unexplained even after an autopsy. Typically, parents put the infant to bed and later find him dead, commonly with no indications of a struggle or distress of any kind. Incidence has decreased with the practice of teaching parents to place an infant on his back to sleep.
CAUSES AND INCIDENCE
SIDS is the third leading cause of death in infants between age 1 month and 1 year. It occurs more commonly in winter months. The incidence is higher in males, preterm neonates, and those who sleep on their stomachs or in cribs with soft bedding. Incidence is also higher among neonates born in conditions of poverty and to those who were one of a single multiple birth, such as twins and triplets, and to mothers who smoke, take drugs, or failed to seek prenatal care until late in the pregnancy. SIDS may also result from an abnormality in the control of ventilation that allows carbon dioxide to build up in the blood, thereby causing prolonged apneic periods with profound hypoxemia and serious cardiac arrhythmias. It’s also thought to be associated with problems in sleep arousal.
SIGNS AND SYMPTOMS
Although parents find some victims wedged in crib corners or with blankets wrapped around their heads, autopsies rule out suffocation as the cause of death. Autopsy shows a patent airway, so aspiration of vomitus isn’t the cause of death. Typically, SIDS babies don’t cry out and show no signs of having been disturbed in their sleep. However, their positions or tangled blankets may suggest movement just before death, perhaps due to terminal spasm.
Depending on how long the infant has been dead, a SIDS baby may have a mottled complexion with extreme cyanosis of the lips and fingertips or pooling of blood in the legs and feet that may be mistaken for bruises. Pulse and respirations are absent, and the diaper is wet and full of stool.
Croup
Croup is a severe inflammation and obstruction of the upper airway, occurring as acute laryngotracheobronchitis (most common), laryngitis, and acute spasmodic laryngitis; it must always be distinguished from epiglottiditis. It’s derived from an old German word for “voice box” and refers to swelling around the larynx or vocal cords. Recovery is usually complete.
CAUSES AND INCIDENCE
Croup usually results from a viral infection but can also be caused by bacteria, allergens, and inhaled irritants. Parainfluenza viruses cause 75% of such infections; adenoviruses, respiratory syncytial virus (RSV), influenza, and measles viruses account for the rest.
Croup is a childhood disease affecting more boys than girls (typically between ages 3 months and 5 years) that usually occurs during the winter. Up to 15% of patients have a strong family history of croup.
SIGNS AND SYMPTOMS
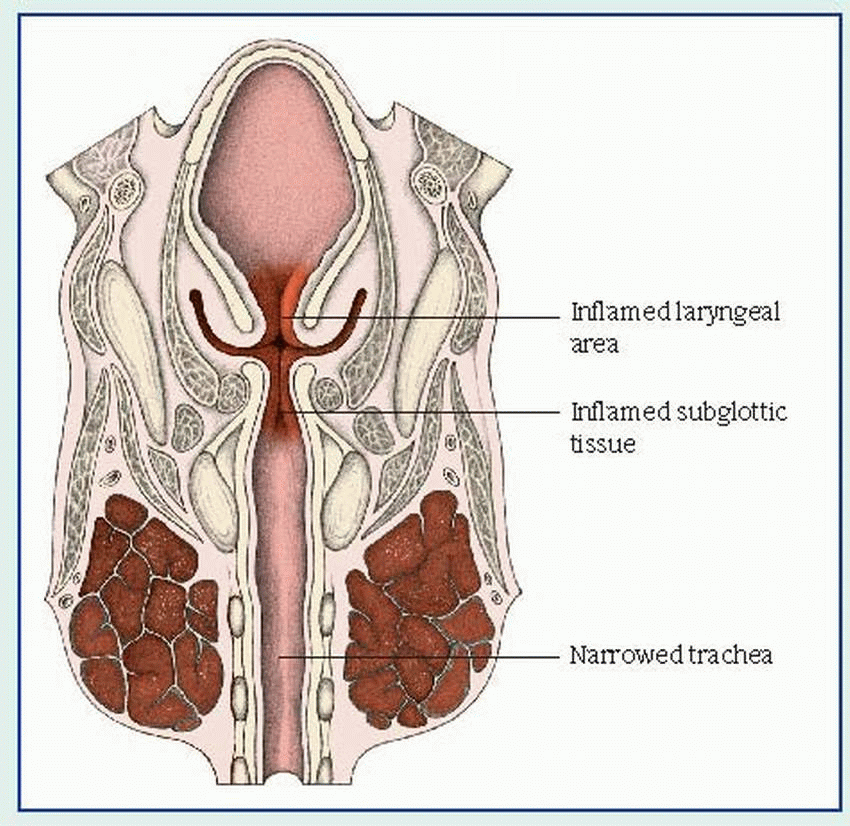
The onset of croup usually follows an upper respiratory tract infection. Clinical features include inspiratory stridor, hoarse or muffled vocal sounds, varying degrees of laryngeal obstruction and respiratory distress, and a characteristic sharp, barking, seal-like cough. These symptoms may last only a few hours or persist for a day or two. As it progresses, croup causes inflammatory edema and, possibly, spasm, which can obstruct the upper airway and severely compromise ventilation. (See
How croup affects the upper airway, page 100.)
Each form of croup has additional characteristics:
In laryngotracheobronchitis, the symptoms seem to worsen at night. Inflammation causes edema of the bronchi and bronchioles as well as increasingly difficult expiration that frightens the child. Other characteristic features include fever, diffusely decreased breath sounds, expiratory rhonchi, and scattered crackles.
Laryngitis, which results from vocal cord edema, is usually mild and produces no respiratory distress except in infants. Early signs include a sore throat and cough, which, rarely, may progress to marked hoarseness, suprasternal and intercostal retractions, inspiratory stridor, dyspnea, diminished breath sounds, restlessness and, in later stages, severe dyspnea and exhaustion.
Acute spasmodic laryngitis affects a child between ages 1 and 3, particularly one with allergies and a family history of croup. It typically begins with mild to moderate hoarseness and nasal discharge, followed by the characteristic cough and noisy inspiration (that usually awaken the child at night), labored breathing with retractions, rapid pulse, and clammy skin. The child understandably becomes anxious, which may lead to increasing dyspnea and transient cyanosis. These severe symptoms diminish after several hours but reappear in a milder form on the next one or two nights.
Epiglottiditis
Acute epiglottiditis, also known as epiglottitis, is an acute inflammation of the epiglottis that tends to cause airway obstruction. A critical emergency, epiglottiditis can prove fatal unless it’s recognized and treated promptly.
CAUSES AND INCIDENCE
Epiglottiditis usually results from infection with Haemophilus influenzae type B (Hib) and, occasionally, pneumococci and group A streptococci. It typically strikes children between ages 2 and 6 years. (However, immunosuppression can predispose adults to epiglottiditis.) Since the advent of the Hib vaccine, epiglottiditis is becoming more rare.
SIGNS AND SYMPTOMS
Sometimes preceded by an upper respiratory infection, epiglottiditis may rapidly progress to complete upper airway obstruction within 2 to 5 hours. Laryngeal obstruction results from inflammation and edema of the epiglottis. Accompanying symptoms include high fever, stridor, sore throat, dysphagia, irritability, restlessness, and drooling. To relieve severe respiratory distress, the child with epiglottiditis may hyperextend his neck, sit up, and lean forward with his mouth open, tongue protruding, and nostrils flaring as he tries to breathe. He may develop inspiratory retractions and rhonchi.