Resection and Reconstruction of Trachea
Joel D. Cooper
Stacey Su
Introduction
The management of tracheal stenosis is a logistic as well as technical exercise requiring the multidisciplinary input of thoracic surgeons, pulmonologists, otolaryngologists, experienced anesthesiologists, and intensivists at different stages of the patient’s care. Patients with acquired tracheal stenosis often present with comorbidities, which require medical optimization prior to surgical resection. In carefully selected patients, however, surgical resection of tracheal stenosis offers a definitive treatment with excellent outcomes and low perioperative risk. The management of tracheal stenosis encompasses diagnosis, initial assessment and management of a critical airway, temporizing maneuvers, and definitive surgical treatment.
There are broadly three different types of tracheal resections, each of which requires a specific operative approach and technique depending on the location and extent of tracheal involvement. The most straightforward of these is a segmental resection with end-to-end anastomosis of a stenosis located in the proximal to mid-trachea. Resections at either end of the trachea, namely a laryngotracheal resection at the proximal end or a carinal resection at the distal end, may require unique release maneuvers, different approaches, and more complicated anatomic procedures.
Etiology
Characterization of tracheal stenosis is based on the etiology of the stenosis, its location and length, whether the stenosis is evolving or mature in nature, and whether the stenosis is limited to a single segment or separate segments in series. Each of these factors is considered in planning the most appropriate intervention.
Benign conditions for which tracheal resection is considered include traumatic injury, inhalational injury, postintubation and posttracheostomy stricture, postintubation tracheoesophageal fistula, and a variety of inflammatory conditions. The most common cause of tracheal stricture is postintubation stenosis, either attributable to prolonged endotracheal intubation or as a complication from a previous tracheostomy (Fig. 1). Idiopathic subglottic stenosis is a disease in young women and typically affects the cricoid and proximal trachea, thus requiring a laryngotracheal resection.
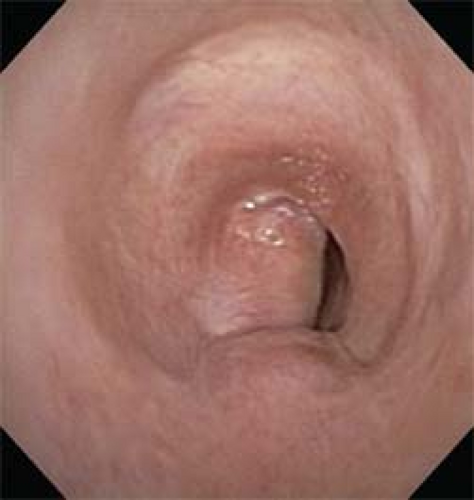 Fig. 1. Bronchoscopic view shows a nearly obliterated upper airway, which may result from postintubation stenosis and high tracheostomies incorrectly placed near the cricoid. |
Airway obstruction from malignant tumors may result from extrinsic compression, local transmural invasion, or endoluminal obstruction. Indications for tracheal resection for malignant tumors include adenoid cystic carcinoma, locally invasive thyroid cancer, and primary squamous carcinoma of the trachea. Adenoid cystic carcinoma is characterized by extensive microscopic involvement of the tracheal wall well beyond the visible borders of involvement. Resection in these cases may be of value even if the final margins show microscopic involvement as subsequent postoperative radiation may be associated with survival of 10 years or more. Resection for primary squamous carcinoma of the trachea is rare due to the advanced stage at the time of diagnosis and likelihood of mediastinal nodal involvement.
Routine preoperative assessment includes a history, physical examination, radiographic imaging, and bronchoscopic evaluation. Whenever possible, patients undergo pulmonary function tests, including a flow-volume loop, which shows a decrease in both maximal inspiratory and expiratory flows. Standard radiographs include anteroposterior and lateral cervical views. A 3D CT reconstruction of the airway yields measurements of the length of the stenosis and its relationship to the rest of the airway (Fig. 2). These measurements serve as a guide before proceeding to more precise confirmation by bronchoscopy. The use of bronchoscopy is essential to show the anatomy of the larynx and glottis, the function of the vocal cords, as well as the configuration of the stenosis and remaining trachea. It is important to note the distance from the stenosis to
anatomic landmarks (vocal cords, carina, and tracheal stoma) in addition to the length of the stenotic segment and the health of the surrounding mucosa.
anatomic landmarks (vocal cords, carina, and tracheal stoma) in addition to the length of the stenotic segment and the health of the surrounding mucosa.
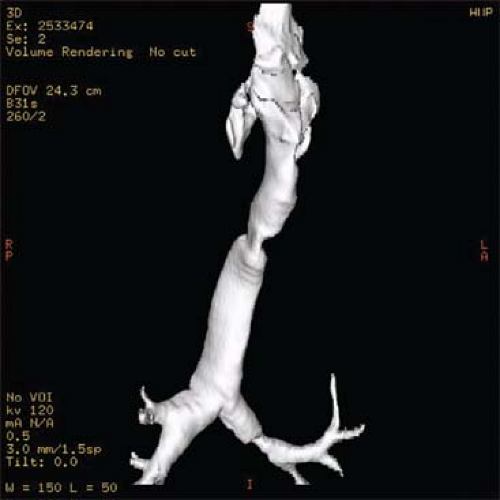 Fig. 2. Three-dimensional CT reconstructions of the airway are useful to assess the configuration of tracheal stenosis before precise measurements are obtained by bronchoscopy. |
The immediate management of the airway once the diagnosis of tracheal stenosis has been made requires as much skill, expertise, and collaboration as the surgical resection itself. There are few indications for emergent tracheal resection, and nearly all cases of tracheal stenosis may be managed initially by establishing a patent airway. After initial evaluation of the anatomy by flexible bronchoscopy (usually through a laryngeal mask airway), dilation across the stenosis may be accomplished by rigid bronchoscopy or by balloon dilation. Balloon dilation may be performed over a Bentson guidewire placed through the working channel of a bronchoscope, with or without the use of fluoroscopy. Especially in the case of critical stenosis, it is important to maintain the patient’s spontaneous breathing and to avoid muscle relaxants until a safe airway has been established. One should be ready with available equipment for an emergent tracheostomy if needed. Rigid bronchoscopy can be performed with bronchoscopes of increasing caliber in order to dilate in a safe, controlled way while maintaining the ability to ventilate, visualize, and suction at the same time.
Most cases of critical stenosis can be initially treated with a single dilation session, the benefits of which lasts 7 to 10 days while an overall plan for long-term management of the airway is formulated. Taking into consideration the patient’s overall medical condition and surgical candidacy, the options include repeated dilation, elective referral for surgical resection, or placement of an airway prosthesis (stent, tracheostomy, or T-tube). If tracheal resection is planned in the ensuing few weeks, dilation alone may be adequate to carry the patient until this time. If internal tracheal stents are to be used, only silicone stents or T-tubes should be utilized since expandable metal stents potentially lead to further mucosal damage and complicate a subsequent surgical resection. In some cases, a T-tube or tracheostomy affords a long-term solution to airway stenosis, either in patients with multiple segments of affected trachea or in poor surgical candidates for resection.
Tracheal Resection
In the last few decades, refinements in the surgical and anesthetic techniques for tracheal resection have yielded excellent outcomes with tolerable morbidity and mortality. Tracheal resection carries multiple benefits. The question of which patient characteristics portend a good outcome requires a consideration of the risk factors for poor outcomes in tracheal resection. As there is seldom a situation which requires emergent tracheal resection and reconstruction, careful patient selection and appropriate timing of surgery are central to successful outcomes of resection. The routine use of steroids and any ongoing requirement for ventilatory support set the stage for a higher rate of anastomotic dehiscence and other complications of tracheal resection. Any patient with reversible medical conditions should have resection deferred until the time at which they are deemed best equipped from a cardiopulmonary standpoint and optimal physical conditioning. Reoperation after a failed tracheal resection is associated with increased risk, complexity, and may produce an inferior result compared to an initial, successful reconstruction.
Principles of Tracheal Resection
The principles of tracheal resection are derived from elementary surgical tenets: a healthy anastomosis will result from meticulous mucosal apposition between well-vascularized tissues opposed without undue tension. To that end, the following corollaries hold: (a) the limits of tracheal resection must extend to healthy, normal tissues, and (b) circumferential dissection beyond the resected ends should be minimized so as to not jeopardize the segmental nature of tracheal blood supply. Up to half of the trachea (about 12 cm) may be resected with primary end-to-end anastomosis. However, if more than three to four cartilaginous rings are resected, then tension-releasing maneuvers beyond flexion of the head and mediastinal mobilization within the avascular pretracheal plane must be considered. Such maneuvers include the suprahyoid release, as described by Montgomery, and the hilar release, with each of these permitting an additional 1 to 2 cm of resection. In general, dissection immediately adjacent to the tracheal wall will prevent injury to the recurrent laryngeal nerves and esophagus. An exception is the posterior cricoid plate, behind which the recurrent laryngeal nerves ascend to enter the cricoarytenoid joint on either side. When the condition to resect back to healthy tissue cannot be met, for example, in many inflammatory conditions where there are multiple affected segments in series or globally inflamed mucosa, then surgical candidacy for tracheal resection must be questioned.
Important Landmarks
The cartilaginous skeleton of the larynx, which houses the vocal cords, is comprised of the thyroid, cricoid, and arytenoid cartilages. The narrowest fixed part of the airway is marked by the cricoid ring. The recurrent laryngeal nerves lie in the tracheoesophageal groove and enter the larynx at the level of the inferior cornu of the thyroid cartilage.
When attempting to excise unhealthy mucosa overlying the posterior cricoid plate, the excision should not be carried >2 cm proximal to the inferior edge of the plate to avoid injury to the cricoarytenoid joint.
When attempting to excise unhealthy mucosa overlying the posterior cricoid plate, the excision should not be carried >2 cm proximal to the inferior edge of the plate to avoid injury to the cricoarytenoid joint.
Incisions
The surgical approach is dictated by the location and extent of diseased trachea. Malignant lesions involving the upper and mid-thirds of the trachea and benign conditions extending down to within four rings of the carina may be managed through a cervical incision. This may require a partial sternotomy for adequate visualization. Malignant lesions involving the distal third and benign conditions extending to within four rings of the carina may be accessed via a right thoracotomy, although a transsternal approach can also be considered. The latter approach allows an exposure of the carina by incising the posterior pericardium while retracting the superior vena cava to the right and the ascending aorta to the left.
Operative Steps
The management of the airway requires synchronized communication between the surgeon and anesthesiologist. At the start of the procedure, assessment by bronchoscopy guides whether initial dilatation with a ventilating scope is required to permit the passage of an orotracheal endotracheal tube beyond the stenosis. In the setting of nearly obstructive lesions, induction via inhaled agents should be employed to preserve spontaneous ventilation. If possible, intubation across the lesion is performed following dilatation. Sterile anesthesia tubing and connectors are passed to the anesthesiologist for use across the operative field. Upon tracheal division, the orotracheal tube is retracted out of the field, and a sterile cuffed 6-0 flexible armored endotracheal tube is intermittently inserted into the cut end of the distal trachea to provide cross-table ventilation. Alternatively, a jet ventilation catheter can be directly placed into the cut distal airway from the field or a separate jet catheter can be advanced from within the lumen of the partially withdrawn oropharyngeal endotracheal tube. Once the posterior half of the anastomotic sutures is complete, the original orotracheal tube is advanced into the distal airway during completion of the procedure.
The steps of tracheal resection and reconstruction can be reduced to the following: (1) localize the diseased segment, (2) mobilize the trachea, (3) transect the trachea, (4) resect the affected area, (5) employ release maneuvers if necessary, and (6) reconstruct.
The patient is placed supine with an inflatable roll beneath the upper back to extend the neck. An esophageal bougie (size 30 Maloney) or nasogastric tube can be placed to facilitate later dissection of the trachea from the esophagus. A transverse cervical incision centered at the cricoid cartilage is made. Skin flaps are elevated in the subplatysmal plane, extending from the superior extent of the larynx to the sternal notch inferiorly. The strap muscles and thyroid isthmus are divided along the midline. Blunt dissection to separate the superficial and deep strap muscles facilitates exposure and subsequent tracheal mobilization. The pretracheal plane is bluntly developed with a finger in the anterior mediastinum toward the carina.
The level of the tracheal stenosis is usually evident through the outward appearance of narrowing, dense scar, and surrounding inflammation. If in doubt, the tracheal stenosis can be localized with the use of a flexible bronchoscope inserted through the endotracheal tube from above. Under bronchoscopic control, the stenosis is then identified by transillumination or by direct localization using a transtracheal 25-gauge needle. The tracheal wall at this point is then marked with a fine stitch. After mobilization is complete, circumferential tracheal dissection at the level of the affected segment is performed sharply, taking care to remain close to the tracheal wall in order to minimize risk of injury to the recurrent laryngeal nerves and to avoid airway devascularization.
Before the trachea is incised, proximal and distal full-thickness 2-0 silk traction sutures are placed 1 to 2 cm away from the proposed line of resection on either side in the mid-lateral position. The trachea is partially transected anteriorly through the stenotic segment (Fig. 3). Subsequent parallel incisions can be made distally until the airway lumen is sufficient to allow direct tracheal intubation and cross-table ventilation. Transection of the posterior wall of the trachea is performed under direct vision from the luminal as well as external aspects, using the esophageal bougie as a guide to help avoid esophageal injury when separating the membranous wall of the airway from the esophagus. The caliber and thickness of the airway wall at the distal extent of the resection is evaluated. If the distal end is not free of disease, then further resection is done, incising 1 to 2 mm at a time in a “breadloaf” fashion to avoid removing normal airway.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


