Objectives
- State the physiological utility of either excreting or reabsorbing organic solutes.
- State the general characteristics of the proximal tubular systems for active reabsorption or secretion of organic solutes.
- Describe the renal handling of glucose and state the conditions under which glycosuria is likely to occur.
- Describe the renal handling of proteins and small peptides.
- Describe the secretion of para-aminohippurate.
- Outline the handling of urate.
- Describe the secretion of organic cations.
- Describe how tubular pH affects the excretion and reabsorption of weak acids and bases.
- Describe the renal handling of urea, including the medullary recycling of urea from the collecting duct to the loop of Henle.
Overview
![]() As pointed out in Chapter 1, a major function of the kidneys is the excretion of organic waste, foreign chemicals and their metabolites. As the kidneys excrete these substances they also filter large amounts of organic substances that they do not excrete, such as glucose and amino acids. Therefore, the kidneys must discriminate between what to keep and what to discard. While the collective concentration of the useful organic solutes that should be kept is small in comparison with inorganic ions like sodium and chloride, the large amounts filtered means that processes must exist to reabsorb them.
As pointed out in Chapter 1, a major function of the kidneys is the excretion of organic waste, foreign chemicals and their metabolites. As the kidneys excrete these substances they also filter large amounts of organic substances that they do not excrete, such as glucose and amino acids. Therefore, the kidneys must discriminate between what to keep and what to discard. While the collective concentration of the useful organic solutes that should be kept is small in comparison with inorganic ions like sodium and chloride, the large amounts filtered means that processes must exist to reabsorb them.
Some organic solutes handled by the kidneys are neutral molecules; most are anions or cations. As the useful metabolites are recovered from the filtrate, the waste and foreign substances are not only let go, but actively secreted. In dealing with organic solutes the kidneys perform a kind of triage. They (1) reabsorb metabolites that should not be lost, (2) eliminate waste products and unwanted foreign organic substances, and (3) partially reabsorb others. An analysis of the renal handling of every one of these organic substances would be prohibitive, so we will discuss a few key solutes and establish generalities about the others.
One organic substance, urea, is unique in this regard. It is a waste product that must be excreted to prevent accumulation. However, it also plays a key role in renal regulation of water balance. The renal handling of urea is briefly discussed later in this chapter and again in the following chapter in the discussion of renal handling of water.
![]() Several generalizations apply to the handling of small organic solutes by the kidney.
Several generalizations apply to the handling of small organic solutes by the kidney.
While there is a strikingly large number of organic solute species, there is a far smaller number of transport protein species, meaning that many transporters are promiscuous, accepting multiple solutes, sometimes over 100 different ones. This allows the kidneys to operate without expressing a separate transporter for each and every solute.
Most organic solutes are transported only in the proximal tubule. Those that are secreted or escape reabsorption in the proximal tubule end up being excreted (an exception, as covered later in this chapter, is when charged species become neutral as a result of changes in tubular pH and are reabsorbed passively in regions beyond the proximal tubule).
Transport involves a cascade of interrelated transport events always beginning with active extrusion of sodium across the basolateral membrane by the Na-K-ATPase. Neutral or negatively charged organic solutes then enter via symporters with sodium, while cations enter via uniporters driven by the negative membrane potential. The resulting intracellular accumulation of the solute in question establishes a favorable gradient for its efflux. The accumulated solutes then leave through a variety of pathways across the opposite membrane from which they entered or couple via an antiporter to the influx of another organic solute.
Proximal Reabsorption of Organic Nutrients
Most of the useful organic nutrients in the plasma that should not be lost in the urine are freely filtered. These include glucose, amino acids, acetate, Krebs cycle intermediates, some water-soluble vitamins, lactate, acetoacetate, β-hydroxybutyrate, and many others. The proximal tubule is the major site for reabsorption of the large quantities of these organic nutrients filtered each day by the renal corpuscles.
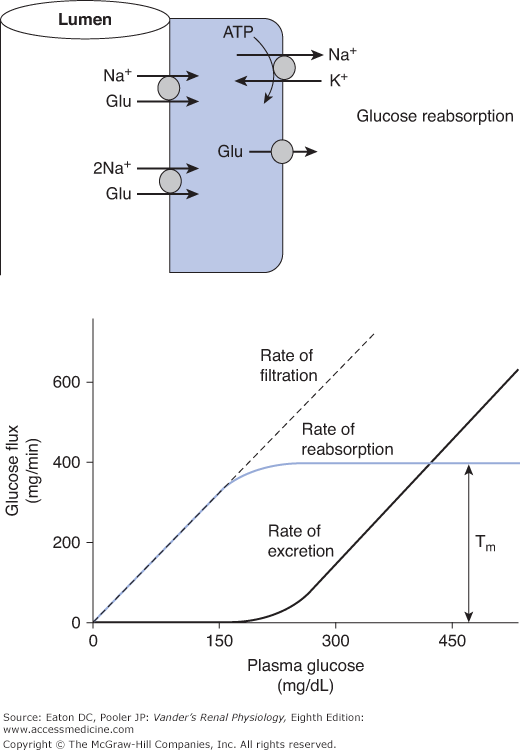
![]() Under most circumstances, it would be deleterious to lose glucose in the urine, particularly in conditions of prolonged fasting. Thus the kidneys normally reabsorb all the glucose that is filtered. A typical plasma glucose level is about 90 mg/dL (5 mmol/L). It rises transiently to well over 100 mg/dL during meals and falls somewhat during fasting. Usually all the filtered glucose is reabsorbed in the proximal tubule. This involves taking up glucose from the tubular lumen across the apical membrane via sodium-glucose symporters, followed by its exit across the basolateral membrane into the interstitium via a GLUT uniporter. Most of the glucose is reabsorbed by a high-capacity, low-affinity sodium-glucose symporter (SGLT-2) that has a stoichiometry of 1 sodium per glucose. Then the last remaining glucose is taken up in the late proximal tubule (S3 segment) by a low-capacity, high-affinity transporter (SGLT-1) that transports 2 sodium ions per glucose (Figure 5–1 top). This 2-for-1 stoichiometry provides additional energy to move glucose up its concentration gradient in the region where the luminal concentration is normally very low. Unlike the case for sodium and many other solutes, the tight junctions are not significantly permeable to glucose. Therefore, as glucose is removed from the lumen and the luminal concentration falls, there is no back-leak, resulting in virtually complete reabsorption.
Under most circumstances, it would be deleterious to lose glucose in the urine, particularly in conditions of prolonged fasting. Thus the kidneys normally reabsorb all the glucose that is filtered. A typical plasma glucose level is about 90 mg/dL (5 mmol/L). It rises transiently to well over 100 mg/dL during meals and falls somewhat during fasting. Usually all the filtered glucose is reabsorbed in the proximal tubule. This involves taking up glucose from the tubular lumen across the apical membrane via sodium-glucose symporters, followed by its exit across the basolateral membrane into the interstitium via a GLUT uniporter. Most of the glucose is reabsorbed by a high-capacity, low-affinity sodium-glucose symporter (SGLT-2) that has a stoichiometry of 1 sodium per glucose. Then the last remaining glucose is taken up in the late proximal tubule (S3 segment) by a low-capacity, high-affinity transporter (SGLT-1) that transports 2 sodium ions per glucose (Figure 5–1 top). This 2-for-1 stoichiometry provides additional energy to move glucose up its concentration gradient in the region where the luminal concentration is normally very low. Unlike the case for sodium and many other solutes, the tight junctions are not significantly permeable to glucose. Therefore, as glucose is removed from the lumen and the luminal concentration falls, there is no back-leak, resulting in virtually complete reabsorption.
Figure 5–1.
Glucose handling by the kidney. (Top) Glucose is taken up across the apical membrane by sodium-glucose symporters and leaves across the basolateral membrane via glucose uniporters (GLUT family). In most of the proximal tubule, the sodium-glucose stoichiometry is 1-for-1 (SGLT-2 isoform). In the late proximal tubule, the stoichiometry is 2-for-1 (SGLT-1 isoform). (Bottom) The rates of filtration, reabsorption, and excretion are plotted as a function of plasma glucose concentration. At a given GFR, the rate of glucose filtration is exactly proportional to the plasma concentration. At normal levels of plasma glucose, this rate is well below the Tm and therefore, all the filtered glucose is reabsorbed and none is excreted. However, as plasma glucose rises into the hyperglycemic range, the Tm is reached and any glucose filtered in excess of the Tm is excreted.
Because the sodium-glucose symporters are saturable (Tm systems), abnormally high-filtered loads overwhelm the reabsorptive capacity (exceed the Tm; Figure 5–1 bottom). This occurs when plasma glucose approaches 200 mg/dL, a situation often found in untreated diabetes mellitus. In very severe cases, plasma glucose can exceed 1000 mg/dL, or over 55 mmol/L, leading to a significant loss of glucose.
Assume that the glucose Tm is 375 mg/min (a typical value). With a glomerular filtration rate (GFR) of 125 mL/min (1.25 dL/min) and a normal plasma glucose of 90 mg/dL, the filtered load is 1.25 dL/min × 90 mg/dL = 112.5 mg/min, well below the Tm of 375 mg/min. Thus the kidneys easily reabsorb the entire filtered load. When plasma glucose reaches 200 mg/dL, the filtered load becomes 1.25 dL/min × 200 mg/dL = 250 mg/min. At this point, some individual nephrons have reached the upper limit of what they can reabsorb, and a little glucose begins to spill into the urine. Further increases in plasma glucose saturate the remaining transporters and any amount filtered above 375 mg/min is excreted. This leads to loss of glucose and an unwanted osmotic diuresis that we discussed in Chapter 4. One can appreciate that any glucose not reabsorbed is an osmole in the tubule that has consequences for water reabsorption.
Proteins and Peptides
![]() Although we sometimes say the glomerular filtrate is protein free, it is not truly free of all protein; it just has a total protein concentration much lower than plasma. First, peptides and smaller proteins (eg, angiotensin, insulin), although present at low concentrations in the blood, are filtered in considerable quantities. Second, while the movement of large plasma proteins across the glomerular filtration barrier is extremely limited, a small amount does make it through into Bowman’s space. For albumin, the plasma protein of highest concentration in the blood, the concentration in the filtrate is normally about 1 mg/dL, or roughly 0.02% of the plasma albumin concentration (5 g/dL). Because of the huge volume of fluid filtered per day, the total filtered amount of protein is not negligible. Normally all of these proteins and peptides are reabsorbed completely, although not in the conventional way. They are enzymatically degraded into their constituent amino acids, which are then returned to the blood.
Although we sometimes say the glomerular filtrate is protein free, it is not truly free of all protein; it just has a total protein concentration much lower than plasma. First, peptides and smaller proteins (eg, angiotensin, insulin), although present at low concentrations in the blood, are filtered in considerable quantities. Second, while the movement of large plasma proteins across the glomerular filtration barrier is extremely limited, a small amount does make it through into Bowman’s space. For albumin, the plasma protein of highest concentration in the blood, the concentration in the filtrate is normally about 1 mg/dL, or roughly 0.02% of the plasma albumin concentration (5 g/dL). Because of the huge volume of fluid filtered per day, the total filtered amount of protein is not negligible. Normally all of these proteins and peptides are reabsorbed completely, although not in the conventional way. They are enzymatically degraded into their constituent amino acids, which are then returned to the blood.
For the larger proteins, the initial step in recovery is endocytosis at the apical membrane. This energy-requiring process is triggered by the binding of filtered protein molecules to specific receptors on the apical membrane. The rate of endocytosis is increased in proportion to the concentration of protein in the glomerular filtrate until a maximal rate of vesicle formation, and thus, the Tm for protein uptake is reached. The pinched-off intracellular vesicles resulting from endocytosis merge with lysosomes, whose enzymes degrade the protein to low-molecular-weight fragments, mainly individual amino acids. These end products then exit the cells across the basolateral membrane into the interstitial fluid, from which they gain entry to the peritubular capillaries.
To understand the potential problem associated with a failure to take up filtered protein, remember that for a healthy young adult
If this protein was not removed from the lumen, the entire 1.8 g would be lost in the urine. In fact, most of the filtered protein is endocytosed and degraded so that the excretion of protein in the urine is normally only 100 mg/day. The endocytic mechanism by which protein is taken up is easily saturated, so a large increase in filtered protein resulting from increased glomerular permeability causes the excretion of large quantities of protein.